
McGraw-Hill Book Co. Inc.
PUBLISHERS OF BOOKS FOR
Coal Age ▼ Electric Railway Journal
Electrical World ▼ Engineering News-Record
American Machinist ▼ Ingeniería Internacional
Engineering & Mining Journal ▼ Power
Chemical & Metallurgical Engineering
Electrical Merchandising

CHEMICAL WARFARE
BY
AMOS A. FRIES
Brigadier General, C. W. S., U. S. A.
Chief, Chemical Warfare Service
AND
CLARENCE J. WEST
Major, C. W. S. Reserve Corps, U. S. A.
National Research Council
First Edition
McGRAW-HILL BOOK COMPANY, Inc.
NEW YORK: 370 SEVENTH AVENUE
LONDON: 6 & 8 BOUVERIE ST., E. C. 4
1921
Copyright, 1921, by the
McGraw-Hill Book Company, Inc.
[Pg vii]
Shortly after the signing of the Armistice, it was realized that the story of Chemical Warfare should be written, partly because of its historical value, and partly because of the future needs of a textbook covering the fundamental facts of the Service for the Army, the Reserve Officer, the National Guard, and even the Civilian Chemist. The present work was undertaken by both authors as a labor of patriotism and because of their interest in the Service.
The two years which have elapsed since the initial discussion of the outlines of the book have thoroughly convinced us of the need of such a work. The Engineers, the Medical Department, and most of the other branches of the Army have their recognized textbooks and manuals. There has been no way, however, by which the uninformed can check the accuracy of statements regarding Chemical Warfare. The present volume will serve, in a measure, to fill this gap. That it does not do so more completely is due in part to the fact that secrecy must still be maintained about some of the facts and some of the new discoveries which are the property of the Service. Those familiar with the work of the Chemical Warfare Service will discover, though, that the following pages contain many statements which were zealously guarded secrets two years ago. This enlarged program of publicity on the part of the Chief of the Service is being justified every day by the ever increasing interest in this branch of warfare. Where five men were discussing Chemical Warfare two years ago, fifty men are talking about the work and the possibilities of the Service today. It is hoped that the facts here presented may further increase the interest in Chemical Warfare, for there is no question but that it must be recognized as a permanent and a very vital branch of the Army of every country. Reasons for this will be found scattered through the pages of this book. [Pg viii]
It should be explained that this is in no sense a complete historical sketch of the development and personnel of the Chemical Warfare Service. At least two more volumes are needed,—one on the Manufacture of Poisonous Gases and one on the Tactics of Chemical Warfare. We have purposely refrained from an attempt to give credit to individuals for the accomplishments of the various Divisions of the Service, because such an attempt would have made the book too voluminous, and would have defeated the primary purpose, namely, that it should present the information in as concise manner as possible. The published and unpublished materials of the files of all the Divisions have been freely drawn upon in writing the various chapters, and many old C. W. S. men will undoubtedly recognize whole sentences which they wrote under the stress of the laboratory or plant “battle front.” May these few lines be an acknowledgment of their contributions. Those who desire to consult the literature of Chemical Warfare will find a fairly complete bibliography (to about the middle of 1919) in “Special Libraries” for November, 1919.
Special acknowledgment is made to Dr. G. J. Esselen, Jr., for having read the manuscript and for helpful and constructive criticisms. Many of the figures are reproduced by permission of the Journal of Industrial and Engineering Chemistry; those showing the Nelson cell were furnished by the Samuel M. Green Company.
Aug. 1, 1921.
[Pg ix]
After all peaceful means of settling disputes between nations have been resorted to and have failed, war is often declared by one of the disputants for the purpose of imposing its will upon the other by force. In order to accomplish this, a superiority must be established over the adversary in trained men and in implements of war.
Men are nothing in modern war unless they are equipped with the most effective devices for killing and maiming the enemy’s soldiers and thoroughly trained in the use of such implements.
History proves that an effective implement of war has never been discarded until it becomes obsolete.
It is impossible to humanize the act of killing and maiming the enemy’s soldiers, and there is no logical grounds on which to condemn an appliance so long as its application can be so confined. Experiments in this and other countries during the World War completely established the fact that gas can be so confined. The range of gas clouds is no greater than that of artillery and the population in the area behind the front line must, if they remain in such range, take their chance. The danger area in the future will be known to all.
As the first Director of the Chemical Warfare Service, U. S. Army, I speak with some experience when I say that there is no field in which the future possibilities are greater than in chemical warfare, and no field in which neglect to keep abreast of the times in research and training would be more disastrous.
Notwithstanding the fact that gas was used in the World War two years before the United States entered the fray, practically nothing was done in this country before April, 1917, towards the development of any chemical warfare appliances, offensive or defensive, and had it not been for the ability of an ally to supply our troops with such [Pg x] appliances, they would have been as defenseless as the Canadians were at Ypres when the Germans sent over their first gas cloud.
This book recites the troubles and successes of this new service under the stress of war for which it was unprepared and I trust that its perusal will create a public opinion that will insist upon chemical preparation for war.
I feel that this book will show that the genius and patriotism displayed by the chemists and chemical engineers of the country were not surpassed in any other branch of war work and that to fail to utilize in peace times this talent would be a crime.
[Pg xi]
CONTENTS
| PAGE | ||
| Preface | vii | |
| Foreword | ix | |
| chapter | ||
| I. | The History of Poison Gases | 1 |
| II. | Modern Development of Gas Warfare | 10 |
| III. | Development of the | |
| Chemical Warfare Service | 31 | |
| IV. | The Chemical Warfare Service in France | 72 |
| V. | Chlorine | 116 |
| VI. | Phosgene | 126 |
| VII. | Lachrymators | 137 |
| VIII. | Chloropicrin | 144 |
| IX. | Dichloroethylsulfide (Mustard Gas) | 150 |
| X. | Arsenic Derivatives | 180 |
| XI. | Carbon Monoxide | 190 |
| XII. | Development of the Gas Mask | 195 |
| XIII. | Absorbents | 237 |
| XIV. | Testing Absorbents and Gas Masks | 259 |
| XV. | Other Defensive Measures | 272 |
| XVI. | Screening Smokes | 285 |
| XVII. | Toxic Smokes | 313 |
| XVIII. | Smoke Filters | 322 |
| XIX. | Signal Smokes | 330 |
| XX. | Incendiary Materials | 336 |
| XXI. | The Pharmacology of War Gases | 353 |
| XXII. | Chemical Warfare in Relation to | |
| Strategy and Tactics | 363 | |
| XXIII. | The Offensive Use of Gas | 385 |
| XXIV. | Defense against Gas | 405 |
| XXV. | Peace Time Uses of Gas | 427 |
| XXVI. | The Future of Chemical Warfare | 435 |
| Index | 440 | |
[Pg 1]
CHEMICAL WARFARE
The introduction of poison gases by the Germans at Ypres in April, 1915, marked a new era in modern warfare. The popular opinion is that this form of warfare was original with the Germans. Such, however, is not the case. Quoting from an article in the Candid Quarterly Review, 4, 561, “All they can claim is the inhuman adoption of devices invented in England, and by England rejected as too horrible to be entertained even for use against an enemy.” But the use of poison gases is even of an earlier origin than this article claims.
The first recorded effort to overcome an enemy by the generation of poisonous and suffocating gases seems to have been in the wars of the Athenians and Spartans (431-404 b.c.) when, besieging the cities of Platea and Belium, the Spartans saturated wood with pitch and sulfur and burned it under the walls of these cities in the hope of choking the defenders and rendering the assault less difficult. Similar uses of poisonous gases are recorded during the Middle Ages. In effect they were like our modern stink balls, but were projected by squirts or in bottles after the manner of a hand grenade. The legend is told of Prester John (about the eleventh century), that he stuffed copper figures with explosives and combustible materials which, emitted from the mouths and nostrils of the effigies, played great havoc.
The idea referred to by the writer in the Candid Quarterly Review, is from the pen of the English Lord Dundonald, which [Pg 2] appeared in the publication entitled “The Panmure Papers.” This is an extremely dull record of an extremely dull person, only rendered interesting by the one portion, concerned with the use of poison gases, which, it is said, “should never have been published at all.”
That portion of the article from the Candid Quarterly Review dealing with the introduction of poisonous gas by the Germans, and referred to in the first paragraph above, is quoted in full as follows:
“The great Admiral Lord Dundonald—perhaps the ablest sea captain ever known, not even excluding Lord Nelson—was also a man of wide observation, and no mean chemist. He had been struck in 1811 by the deadly character of the fumes of sulphur in Sicily; and, when the Crimean War was being waged, he communicated to the English government, then presided over by Lord Palmerston, a plan for the reduction of Sebastopol by sulphur fumes. The plan was imparted to Lord Panmure and Lord Palmerston, and the way in which it was received is so illustrative of the trickery and treachery of the politician that it is worth while to quote Lord Palmerston’s private communication upon it to Lord Panmure:
“Lord Palmerston to Lord Panmure
“‘House of Commons, 7th August, 1855 “‘I agree with you that if Dundonald will go out himself to superintend and direct the execution of his scheme, we ought to accept his offer and try his plan. If it succeeds, it will, as you say, save a great number of English and French lives; if it fails in his hands, we shall be exempt from blame, and if we come in for a small share of the ridicule, we can bear it, and the greater part will fall on him. You had best, therefore, make arrangement with him without delay, and with as much secrecy as the nature of things will admit of.’
“Inasmuch as Lord Dundonald’s plans have already been deliberately published by the two persons above named, there can be no harm in now republishing them. They will be found in the first volume of ‘The Panmure Papers’ (pp. 340-342) and are as follows:
[Pg 3]
“‘(Enclosure)
“‘Brief Preliminary Observations
“‘It was observed when viewing the Sulphur Kilns, in July, 1811, that the fumes which escaped in the rude process of extracting the material, though first elevated by heat, soon fell to the ground, destroying all vegetation, and endangering animal life to a great distance, and it was asserted that an ordinance existed prohibiting persons from sleeping within the distance of three miles during the melting season.
“‘An application of these facts was immediately made to Military and Naval purposes, and after mature consideration, a Memorial was presented on the subject to His Royal Highness the Prince Regent on the 12th of April, 1812, who was graciously pleased to lay it before a Commission, consisting of Lord Keith, Lord Exmouth and General and Colonel Congreve (afterwards Sir William), by whom a favorable report having been given, His Royal Highness was pleased to order that secrecy should be maintained by all parties.
“‘7th August, 1855’
“‘Memorandum
“‘Materials required for the expulsion of the Russians from Sebastopol: Experimental trials have shown that about five parts of coke effectually vaporize one part of sulphur. Mixtures for land service, where weight is of importance, may, however, probably be suggested by Professor Faraday, as to operations on shore I have paid little attention. Four or five hundred tons of sulphur and two thousand tons of coke would be sufficient.
“‘Besides these materials, it would be necessary to have, say, as much bituminous coal, and a couple of thousand barrels of gas or other tar, for the purpose of masking fortifications to be attacked, or others that flank the assailing positions.
“‘A quantity of dry firewood, chips, shavings, straw, hay or other such combustible materials, would also be requisite quickly to kindle the fires, which ought to be kept in readiness for the first favourable and steady breeze.
“‘7th August, 1855’
[Pg 4]
“‘Note.—The objects to be accomplished being specially stated the responsibility of their accomplishment ought to rest on those who direct their execution.
“‘Suppose that the Malakoff and Redan are the objects to be assailed it might be judicious merely to obscure the Redan (by the smoke of coal and tar kindled in ‘The Quarries’), so that it could not annoy the Mamelon, where the sulphur fire would be placed to expel the garrison from the Malakoff, which ought to have all the cannon that can be turned towards its ramparts employed in overthrowing its undefended ramparts.
“‘There is no doubt but that the fumes will envelop all the defenses from the Malakoff to the Barracks, and even to the line of battleship, the Twelve Apostles, at anchor in the harbour.
“‘The two outer batteries, on each side of the Port, ought to be smoked, sulphured, and blown down by explosion vessels, and their destruction completed by a few ships of war anchored under cover of the smoke.’
“That was Lord Dundonald’s plan in 1855, improperly published in 1908, and by the Germans, who thus learnt it, ruthlessly put into practise in 1915.
“Lord Dundonald’s memoranda, together with further elucidatory notes, were submitted by the English government of that day to a committee and subsequently to another committee in which Lord Playfair took leading part. These committees, with Lord Dundonald’s plans fully and in detail before them, both reported that the plans were perfectly feasible; that the effects expected from them would undoubtedly be produced; but that those effects were so horrible that no honorable combatant could use the means required to produce them. The committee therefore recommended that the scheme should not be adopted; that Lord Dundonald’s account of it should be destroyed. How the records were obtained and preserved by those who so improperly published them in 1908 we do not know. Presumably they were found among Lord Panmure’s papers. Admiral Lord Dundonald himself was certainly no party to their publication.”
One of the early, if not the earliest suggestion as to the use of poison gas in shell is found in an article on “Greek Fire,” by B. W. Richardson.[2] He says:[Pg 5]
“I feel it a duty to state openly and boldly, that if science were to be allowed her full swing, if society would really allow that ‘all is fair in war,’ war might be banished at once from the earth as a game which neither subject nor king dare play at. Globes that could distribute liquid fire could distribute also lethal agents, within the breath of which no man, however puissant, could stand and live. From the summit of Primrose Hill, a few hundred engineers, properly prepared, could render Regent’s Park, in an incredibly short space of time, utterly uninhabitable; or could make an army of men, that should even fill that space, fall with their arms in their hands, prostrate and helpless as the host of Sennacherib.
“The question is, shall these things be? I do not see that humanity should revolt, for would it not be better to destroy a host in Regent’s Park by making the men fall as in a mystical sleep, than to let down on them another host to break their bones, tear their limbs asunder and gouge out their entrails with three-cornered pikes; leaving a vast majority undead, and writhing for hours in torments of the damned? I conceive, for one, that science would be blessed in spreading her wings on the blast, and breathing into the face of a desperate horde of men prolonged sleep—for it need not necessarily be a death—which they could not grapple with, and which would yield them up with their implements of murder to an enemy that in the immensity of its power could afford to be merciful as Heaven.
“The question is, shall these things be? I think they must be. By what compact can they be stopped? It were improbable that any congress of nations could agree on any code regulating means of destruction; but if it did, it were useless; for science becomes more powerful as she concentrates her forces in the hands of units, so that a nation could only act, by the absolute and individual assent of each of her representatives. Assume, then, that France shall lay war to England, and by superior force of men should place immense hosts, well armed, on English soil. Is it probable that the units would rest in peace and allow sheer brute force to win its way to empire? Or put English troops on French soil, and reverse the question?
“To conclude. War has, at this moment, reached, in its details, such an extravagance of horror and cruelty, that it can not be made worse by any art, and can only be made more merciful by being rendered more terribly energetic. Who that had to die from a blow would not rather place his head under Nasmyth’s hammer, than submit it to a drummer-boy armed with a ferrule?”
The Army and Navy Register of May 29, 1915, reports that [Pg 6]
“among the recommendations forwarded to the Board of Ordnance and Fortifications there may be found many suggestions in favor of the asphyxiation process, mostly by the employment of gases contained in bombs to be thrown within the lines of the foe, with varying effects from peaceful slumber to instant death. One ingenious person suggested a bomb laden to its full capacity with snuff, which should be so evenly and thoroughly distributed that the enemy would be convulsed with sneezing, and in this period of paroxysm it would be possible to creep up on him and capture him in the throes of the convulsion.”
That the probable use of poisonous gas has often been in the minds of military men during recent times is evidenced by the fact that at the Hague Conference in 1899 several of the more prominent nations of Europe and Asia pledged themselves not to use projectiles whose only object was to give out suffocating or poisonous gases. Many of the Powers did not sign this declaration until later. Germany signed and ratified it on Sept. 4, 1900, but the United States never signed it. Further, this declaration was not to be binding in case of a war in which a non-signatory was or became a belligerent. Admiral Mahan, a United States delegate, stated his position in regard to the use of gas in shell (at that time an untried theory) as follows:
“The reproach of cruelty and perfidy addressed against these supposed shells was equally uttered previously against fire-arms and torpedoes, although both are now employed without scruple. It is illogical and not demonstrably humane to be tender about asphyxiating men with gas, when all are prepared to admit that it is allowable to blow the bottom out of an ironclad at midnight, throwing four or five hundred men into the sea to be choked by the water, with scarcely the remotest chance to escape.”
At the Hague Congress of 1907, article 23 of the rules adopted for war on land states:
“It is expressly forbidden (a), to employ poisons or poisonous weapons.”
Before the War suffocating cartridges were shot from the cartridge-throwing rifle of 26 mm. These cartridges were charged with ethyl bromoacetate, a slightly suffocating and non-toxic lachrymator. They were intended for attack on the flanking works of permanent fortifications, flanking casements or caponiers, into which the enemy [Pg 7] tried to make the cartridges penetrate through the narrow slits used for loopholes. The men who were serving the machine guns or the cannon of the flanking works would have been bothered by the vapor from the ethyl bromoacetate, and the assailant would have profited by their disturbance to get past the obstacle presented by the fortification. The employment of these devices, not entailing death, did not contravene the Hague conventions.
The only memorable operations in the course of which these devices were used before the War was the attack on the Bonnet gang at Choisy-le-roi.
In connection with the suggested use of sulfur dioxide by Lord Dundonald and the proposed use of poisonous gases in shell, the following description of a charcoal respirator by Dr. J. Stenhouse,[3] communicated by Dr. George Wilson in 1854, is of interest.
“Dr. Wilson commenced by stating that, having read with much interest the account of Dr. Stenhouse’s researches on the deodorizing and disinfecting properties of charcoal, and the application of these to the construction of a new and important kind of respirator, he had requested the accomplished chemist to send one of his instruments for exhibition to the society, which he had kindly done. Two of the instruments were now on the table, differing, however, so slightly in construction, that it would be sufficient to explain the arrangement of one of them. Externally, it had the appearance of a small fencing-mask of wire gauze, covering the face from the chin upwards to the bridge of the nose, but leaving the eyes and forehead free. It consisted, essentially, of two plates of wire gauze, separated from each other by a space of about one-fourth or one-eighth of an inch, so as to form a small cage filled with small fragments of charcoal. The frame of the cage was of copper, but the edges were made of soft lead, and were lined with velvet, so as to admit of their being made to fit the cheeks tightly and inclose the mouth and nostrils. By this arrangement, no air could enter the lungs without passing through the wire gauze and traversing the charcoal. An aperture is provided with a screw or sliding valve for the removal and replenishment of the contents of the cage, which consist of the siftings or riddlings of the lighter kinds of wood charcoal. The apparatus is attached to the face by an elastic [Pg 8] band passing over the crown of the head and strings tying behind, as in the case of the ordinary respirator. The important agent in this instrument is the charcoal, which has so remarkable a power of absorbing and destroying irritating and otherwise irrespirable and poisonous gases or vapors that, armed with the respirator, spirits of hartshorn, sulphuretted hydrogen, hydrosulphuret of ammonia and chlorine may be breathed through it with impunity, though but slightly diluted with air. This result, first obtained by Dr. Stenhouse, has been verified by those who have repeated the trial, among others by Dr. Wilson, who has tried the vapors named above on himself and four of his pupils, who have breathed them with impunity. The explanation of this remarkable property of charcoal is two-fold. It has long been known to possess the power of condensing into its pores gases and vapors, so that if freshly prepared and exposed to these, it absorbs and retains them. But it has scarcely been suspected till recently, when Dr. Stenhouse pointed out the fact, that if charcoal be allowed to absorb simultaneously such gases as sulphuretted hydrogen and air, the oxygen of this absorbed and condensed air rapidly oxidizes and destroys the accompanying gas. So marked is this action, that if dead animals be imbedded in a layer of charcoal a few inches deep, instead of being prevented from decaying as it has hitherto been supposed that they would be by the supposed antiseptic powers of the charcoal, they are found by Dr. Stenhouse to decay much faster, whilst at the same time, no offensive effluvia are evolved. The deodorizing powers of charcoal are thus established in a way they never have been before; but at the same time it is shown that the addition of charcoal to sewage refuse lessens its agricultural value contemporaneously with the lessening of odor. From these observations, which have been fully verified, it appears that by strewing charcoal coarsely powdered to the extent of a few inches, over church-yards, or by placing it inside the coffins of the dead, the escape of noisome and poisonous exhalations may be totally prevented. The charcoal respirator embodies this important discovery. It is certain that many of the miasma, malaria and infectious matters which propagate disease in the human subjects, enter the body by the lungs, and impregnating the blood there, are carried with it throughout the entire body, which they thus poison. These miasma are either gases and vapors or bodies which, like fine light dust, are readily carried through the air; moreover, they are readily destroyed by oxidizing agents, which convert them into harmless, or at least non-poisonous substances, such as water, carbonic acid and nitrogen. There is every reason, therefore, for believing that charcoal will oxidize and destroy such miasma as effectually as it does [Pg 9] sulphuretted hydrogen or hydrosulphuret of ammonia, and thus prevent their reaching and poisoning the blood. The intention accordingly is that those who are exposed to noxious vapors, or compelled to breathe infected atmospheres, shall wear the charcoal respirator, with a view to arrest and destroy the volatile poisons contained in these. Some of the non-obvious applications of the respirator were then referred to:
“1. Certain of the large chemical manufacturers in London are now supplying their workmen with the charcoal respirators as a protection against the more irritating vapors to which they are exposed.
“2. Many deaths have occurred among those employed to explore the large drains and sewers of London from exposure to sulphuretted hydrogen, etc. It may be asserted with confidence that fatal results from exposure to the drainage gases will cease as soon as the respirator is brought into use.
“3. In districts such as the Campagna of Rome, where malaria prevails and to travel during night or to sleep in which is certainly followed by an attack of dangerous and often fatal ague, the wearing of the respirator even for a few hours may be expected to render the marsh poison harmless.
“4. Those, who as clergymen, physicians or legal advisers, have to attend the sick-beds of sufferers from infectious disorders, may, on occasion, avail themselves of the protection afforded by Dr. Stenhouse’s instrument during their intercourse with the sick.
“5. The longing for a short and decisive war has led to the invention of ‘a suffocating bombshell,’ which on bursting, spreads far and wide an irrespirable or poisonous vapor; one of the liquids proposed for the shell is the strongest ammonia, and against this it is believed that the charcoal respirator may defend our soldiers. As likely to serve this end, it is at present before the Board of Ordnance.
“Dr. Wilson stated, in conclusion, that Dr. Stenhouse had no interest but a scientific one in the success of the respirators. He had declined to patent them, and desired only to apply his remarkable discoveries to the abatement of disease and death. Charcoal had long been used in filters to render poisonous water wholesome; it was now to be employed to filter poisonous air.”
[Pg 10]
The use of toxic gas in the World War dates from April 22, 1915, when the Germans launched the first cylinder attack, employing chlorine, a common and well known gas. Judging from the later experience of the Allies in perfecting this form of attack, it is probable that plans for this attack had been under way for months before it was launched. The suggestion that poisonous gases be used in warfare has been laid upon Prof. Nernst of the University of Berlin (Auld, “Gas and Flame,” page 15), while the actual field operations were said to have been under the direction of Prof. Haber of the Kaiser Wilhelm Physical Chemical Institute of Berlin. Some writers have felt that the question of preparation had been a matter of years rather than of months, and refer to the work on industrial gases as a proof of their statement. The fact that the gas attack was not more successful, that the results to be obtained were not more appreciated, and that better preparation against retaliation had not been made, argues against this idea of a long period of preparation, except possibly in a very desultory way. That such was the case is most fortunate for the allied cause, for had the German high command known the real situation at the close of the first gas attack, or had that attack been more severe, the outcome of the war of 1914 would have been very different, and the end very much earlier.
The first suggestion of a gas attack came to the British Army through the story of a German deserter. He stated that the German Army was planning to poison their enemy with a cloud of gas, and that the [Pg 11] cylinders had already been installed in the trenches. No one listened to the story, because, first of all, the whole procedure seemed so impossible and also because, in spite of the numerous examples of German barbarity, the English did not believe the Germans capable of such a violation of the Hague rules of warfare. The story appeared in the summary of information from headquarters (“Comic Cuts”) and as Auld says “was passed for information for what it is worth.” But the story was true, and on the afternoon of the 22nd of April, all the conditions being ideal, the beginning of “gas warfare” was launched. Details of that first gas attack will always be meager, for the simple reason that the men who could have told about it all lie in Flanders field where the poppies grow.
The place selected was in the northeast part of the Ypres salient, at that part of the line where the French and British lines met, running southward from where the trenches left the canal near Boesinghe. The French right was held by the —— Regiment of Turcos, while on the British left were the Canadians. Auld describes the attack as follows:
“Try to imagine the feelings and the condition of the colored troops as they saw the vast cloud of greenish-yellow gas spring out of the ground and slowly move down wind towards them, the vapor clinging to the earth, seeking out every hole and hollow and filling the trenches and shell holes as it came. First wonder, then fear; then, as the first, fringes of the cloud enveloped them and left them choking and agonized in the fight for breath—panic. Those who could move broke and ran, trying, generally in vain, to outstrip the cloud which followed inexorably after them.”
It is only to be expected that the first feeling connected with gas warfare was one of horror. That side of it is very thrillingly described by Rev. O. S. Watkins in the Methodist Recorder (London). After describing the bombardment of the City of Ypres from April 20th to 22nd he relates that in the midst of the uproar came the poison gas! [Pg 12]

Fig. 1.—French Gas Attack as seen from an Aeroplane.
The French front, second and third line trenches are plainly visible.
The gas is seen issuing over a wide front from the front line and
drifting towards the German lines.
[Pg 13]
“Going into the open air for a few moments’ relief from the stifling atmosphere of the wards, our attention was attracted by very heavy firing to the north, where the line was held by the French. Evidently a hot fight—and eagerly we scanned the country with our field glasses hoping to glean some knowledge of the progress of the battle. Then we saw that which almost caused our hearts to stop beating—figures running wildly and in confusion over the fields.
“‘The French have broken,’ we exclaimed. We hardly believed our words.... The story they told we could not believe; we put it down to their terror-stricken imaginings—a greenish-gray cloud had swept down upon them, turning yellow as it traveled over the country, blasting everything it touched, shriveling up the vegetation. No human courage could face such a peril.
“Then there staggered into our midst French soldiers, blinded, coughing, chests heaving, faces an ugly purple color—lips speechless with agony, and behind them, in the gas-choked trenches, we learned that they had left hundreds of dead and dying comrades. The impossible was only too true.
“It was the most fiendish, wicked thing I have ever seen.”
It must be said here, however, that this was true only because the French had no protection against the gas. Indeed, it is far from being the most horrible form of warfare, provided both sides are prepared defensively and offensively. Medical records show that out of every 100 Americans gassed less than two died, and as far as records of four years show, very few are permanently injured. Out of every 100 American casualties from all forms of warfare other than gas more than 25 per cent died, while from 2 to 5 per cent more are maimed, blinded or disfigured for life. Various forms of gas, as will be shown in the following pages, make life miserable or vision impossible to those without a mask. Yet they do not kill.
Thus instead of gas warfare being the most horrible, it is the most humane where both sides are prepared for it, while against savage or unprepared peoples it can be made so humane that but very few casualties will result.
The development of methods of defense against gas will be discussed in a later chapter. It will suffice to say here that, in response to an appeal from Lord Kitchener, a temporary protection was quickly furnished the men. This was known as the “Black Veiling” respirator, and consisted of a cotton pad soaked in ordinary washing soda solution, and later, in a mixture of washing soda and “hypo,” to which was added [Pg 14] a little glycerine. These furnished a fair degree of protection to the men against chlorine, the only gas used in the early attacks.
The use of chlorine alone continued until the introduction on December 19, 1915, of a mixture of phosgene with the chlorine. This mixture offered many advantages over the use of chlorine alone (see Chapter VI).
The Allies were able, through warning of the impending use of phosgene, to furnish a means of protection against it. It was at this time that the P and the PH helmets were devised, the cotton filling being impregnated with sodium phenolate and later with a mixture of sodium phenolate and hexamethylenetetramine. This helmet was used until the Standard Box Respirator was developed by the late Lt. Col. Harrison.
For a week or two the Allies were very hesitant about adopting gas warfare. However, when the repeated use of gas by the Germans made it evident that, in spite of what the Hague had to say about the matter, gas was to be a part, and as later developments showed, a very important part of modern warfare, they realized there was no choice on their part and that they had to retaliate in like manner. This decision was reached in May of 1915. It was followed by the organization of a Gas Service and intensive work on the part of chemists, engineers and physiologists. It was September 25, 1915, however, before the English were in a position to render a gas attack. From then on the Service grew in numbers and in importance, whether viewed from the standpoint of research, production, or field operations.
The Allies of course adopted not only chlorine but phosgene as well, since both were cheap, easy of preparation and effective. They felt during the early part of the War that they should adopt a substance that would kill instantly, and not one that would cause men to suffer either during the attack or through symptoms which would develop later in a hospital. For this reason a large amount of experimental work was [Pg 15] carried out on hydrocyanic acid, particularly by the French. Since this gas has a very low density, it was necessary to mix with it substances which would tend to keep it close to the ground during the attack. Various mixtures, all called “vincennite,” were prepared,—chloroform, arsenic trichloride and stannic chloride being used in varying proportions with the acid. It was some time before it was definitely learned that these mixtures were far from being successful, both from the standpoint of stability and of poisonous properties. While the French actually used these mixtures in constantly decreasing quantities on the field for a long time, they were ultimately abandoned, though not until American chemists had also carried out a large number of tests. However, following the recommendation of the American Gas Service in France in December, 1917, no vincennite was ever manufactured by the United States.
Almost simultaneously with the introduction of the gas wave attacks, in which liquefied gas under pressure was liberated from cylinders, came the use of lachrymatory or tear gases. These, while not very poisonous in the concentrations used, were very effective in incapacitating men through the effects produced upon their eyes. The low concentration required (one part in ten million of some lachrymators is sufficient to make vision impossible without a mask) makes this form of gas warfare very economical as well as very effective. Even if a mask does completely protect against such compounds, their use compels an army to wear the mask indefinitely, with an expenditure of shell far short of that required if the much more deadly gases were used. Thus Fries estimates that one good lachrymatory shell will force wearing the mask over an area that would require 500 to 1000 phosgene shell of equal size to produce the same effect. While the number of actual casualties will be very much lower, the total effect considered from the standpoint of the expenditure of ammunition and of the objectives gained, will be just as valuable. So great is the harassing value of [Pg 16] tear and irritant gases that the next war will see them used in quantities approximating that of the more poisonous gases.
The first lachrymator used was a mixture of the chlorides and bromides of toluene. Benzyl chloride and bromide are the only valuable substances in this mixture, the higher halogenated products having little or no lachrymatory value. Xylyl bromide is also effective. Chloroacetone and bromoacetone are also well known lachrymators, though they are expensive to manufacture and are none too stable. Because of this the French modified their preparation and obtained mixtures to which they gave the name “martonite.” This is a mixture of 80 per cent bromoacetone and 20 per cent chloroacetone, and can be made with nearly complete utilization of the halogen. Methyl ethyl ketone may also be used, which gives rise to the “homomartonite” of the French. During the early part of the War, when bromine was so very expensive, the English developed ethyl iodoacetate. This was used with or without the addition of alcohol. Later the French developed bromobenzyl cyanide, C₆H₅CH(Br)CN. This was probably the best lachrymator developed during the War and put into large scale manufacture, though very little of it was available on the field of battle before the War ended. Chloroacetophenone would have played an important part had the War continued.
As will be discussed more fully in the chapters on “The Tactics of Gas,” the wave attacks became relatively less important in 1916 through the use of gas in artillery shell. This was the result of many factors. Cloud gas attacks, as carried out under the old conditions, required a long time for the preliminary preparations, entailed a great deal of labor under the most difficult conditions, and were dangerous of execution even when weather conditions became suitable. The difficulties may be summarized as follows:
(1) The heavy gas cylinders used required a great deal of transportation, and not only took the time of the Infantry but rendered [Pg 17] surprise attacks difficult owing both to the time required and to the unusual activity behind the lines that became, with the development of aeroplanes, more and more readily discerned.
(2) Few gases were available for wave attacks—chlorine, phosgene and, to a less extent, chloropicrin proving to be the only ones successfully used by either the Allies or the Germans. Hydrogen sulfide, carbon monoxide and hydrocyanic acid gas were suggested and tried, but were abandoned for one reason or another.
(3) Gas cloud attacks were wholly dependent upon weather conditions. Not only were the velocity and direction of the wind highly important as regards the successful carrying of the wave over the enemy’s line, but also to prevent danger to the troops making the attack due to a possible shift of the wind, which would carry the gas back over their own line.
(4) The use of gas in artillery shell does not require especially trained troops inasmuch as gas shell are fired in the same manner as ordinary shell, and by the same gun crews. Moreover, since artillery gas shell are used generally only for ranges of a mile or more, the direction and velocity of the wind are of minor importance. Another factor which adds to the advantage of artillery shell in certain cases is the ability to land high concentrations of gas suddenly upon a distant target through employing a large number of the largest caliber guns available for firing gas.
Notwithstanding the above named disadvantages of wave attacks it was felt by the Americans from the beginning that successful gas cloud attacks were so fruitful in producing casualties and were such a strain upon those opposed to it, that they would continue. Furthermore, since artillery shell contain about 10 per cent gas, while gas cylinders may contain 50 per cent, or even more of the total weight of the cylinder, the efficiency of a cloud gas attack for at least the first mile of the enemy’s territory is far greater than that of the artillery gas attack. It was accordingly felt that the only thing necessary to make cloud gas attacks highly useful and of frequent occurrence in the future was the development of mobile methods—methods whereby the gas attack could be [Pg 18] launched on the surface of the ground and at short notice. For these reasons gas wave attacks may be expected to continue and to eventually reach a place of very decided importance in Chemical Warfare.
The firing of gas in artillery shell and in bombs has another great advantage over the wave attack just mentioned. There is a very great latitude in the choice of those gases which have a high boiling point or which, at ordinary temperatures, are solids. Mustard gas is an example of a liquid with a high boiling point, and diphenylchloroarsine an example of a gas that is ordinarily solid. For the above reason the term “gas warfare” was almost a misnomer at the close of the War, and today is true only in the sense that all the substances used are in a gaseous or finely divided condition immediately after the shell explode or at least when they reach the enemy.
Still another method of attack, developed by the British and first used by them in July, 1917, was the projector (invented by Captain Livens). This was used very successfully up to the close of the War, and though the German attempted to duplicate it, his results were never as effective. The projector consists of a steel tube of uniform cross section, with an internal diameter of about 8 inches. By using nickel steel the weight may be decreased until it is a one man load. The projector was set against a pressed steel base plate (about 16 inches in diameter) placed in a very shallow trench. [Pg 19]

Fig. 2.—Livens’ Projector.
The Type shown is an 18 cm. German Gas Projector,
captured during the 2d Battle of the Marne.
Until about the close of the war projectors were installed by digging a triangular trench deep enough to bring the muzzles of the projectors nearly level with the surface of the ground. They were then protected by sand bags or canvas covers, or camouflaged with wire netting to which colored bits of cloth were tied to simulate leaves and shadows. The projectors were fired by connecting them in series with ordinary blasting machines operated by hand from a convenient point in the rear. The digging in of the projectors in No Man’s Land or very close to it was a dangerous and laborious undertaking. The Americans early conceived the idea that projectors could be fired just as accurately by digging a shallow trench just deep enough to form a support for the base plate, and then supporting the outer ends of the projector on crossed sticks or a light frame work of boards. This idea proved entirely practical except for one condition. It was found necessary to fire with a single battery all the projectors near enough together to be disturbed by the blast from any portion of them. Inasmuch as most of the blasting machines used for firing had a capacity of only 20 to 30 projectors, it was necessary to so greatly scatter a large projector [Pg 20] attack that the method was very little used. However, investigations were well under way at the close of the War to develop portable firing batteries that would enable the discharge of at least 100 and preferably 500 projectors at one time. By this arrangement a projector attack could be prepared and launched in two to four hours, depending upon the number of men available. This enabled the attack to be decided upon in the evening (if the weather conditions were right), and to have the attack launched before morning, thereby making it impossible for aeroplane observers, armed with cameras, to discover the preparation for the projector attack. Since the bombs used in the projector may carry as high as 30 pounds of gas (usually phosgene), some idea of the amount of destruction may be gained when it is known that the British fired nearly 2500 at one time into Lens.
Another British invention is the Stokes’ gun or trench mortar. The range of this gun is about 800 to 1000 yards. It is therefore effective only where the front lines are relatively close together. The shell consists of a case containing the high explosive, smoke material or gas, fitted to a base filled with a high charge of propelling powder. The shell is simply dropped into the gun. At the bottom of the gun there is a projection or stud that strikes the primer, setting off the small charge and expelling the projectile. In order to obtain any considerable concentration of gas in a particular locality, it is necessary to fire the Stokes’ continuously (15 shots per minute being possible under battle conditions) for two to five minutes since the bomb contains only seven pounds of gas.
It is believed that the first gas shell contained lachrymators or tear gases. Although the use of these shell continued up to and even after the introduction of mustard gas, they gradually fell off in number—the true poison gas shell taking their place. Towards the end of 1915 Auld [Pg 21] states that the Germans were using chloromethyl chloroformate (palite) in shell. In 1916, during the battle of the Somme, palite was replaced by superpalite (trichloromethyl chloroformate, or diphosgene) which is more toxic than palite, and about as toxic as phosgene. It has the advantage over phosgene of being much more persistent. In spite of the fact that American chemists were not able to manufacture superpalite on a large scale, or at least so successfully that it would compete in price with other war gases, the Germans used large quantities of it, alone and mixed with chloropicrin, in shell of every caliber up to and including the 15 cm. Howitzer.
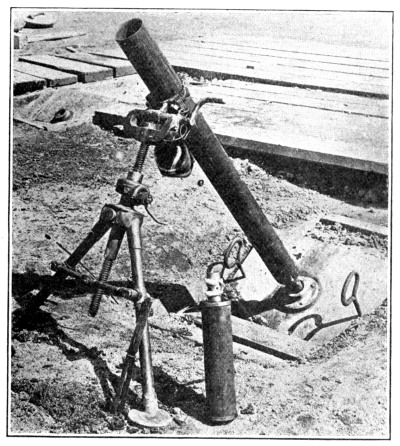
Fig. 3.—Stokes’ Mortar.
The next gas to be introduced was chloropicrin, trichloronitromethane or “vomiting gas.” It has been stated that a mixture of chloropicrin [Pg 22] (25 per cent) and chlorine (75 per cent) has been used in cloud attacks, but the high boiling point of chloropicrin (112° C.) makes its considerable use for this purpose very unlikely. The gas is moderately toxic and somewhat lachrymatory, but it was mainly used because of its peculiar property of causing vomiting when inhaled. Its value was further increased at first because it was particularly difficult to prepare a charcoal which would absorb it. Its peculiar properties are apt to cause it to be used for a long time.
During the summer of 1917 two new and very important gases were introduced, and, as before, by the Germans. One of these was diphenylchloroarsine, “sneezing gas” or “Blue Cross.” This is a white solid which was placed in a bottle and embedded in TNT in the shell. Upon explosion of the shell the solid was atomized into very fine particles. Since the ordinary mask does not remove smoke or mists, the sneezing gas penetrates the mask and causes violent sneezing. The purpose, of course, is to compel the removal of the mask in an atmosphere of lethal gas. (The firing regulations prescribed its use with phosgene or other lethal shell.) The latest type masks protect against this dust, but as it is extraordinarily powerful, its use will continue.
The second gas was dichloroethyl sulfide, mustard gas, Yellow Cross or Yperite. Mustard gas, as it is commonly designated, is probably the most important single poisonous substance used in gas warfare. It was first used by the Germans at Ypres, July 12, 1917. The amount of this gas used is illustrated by the fact that at Nieuport more than 50,000 shell were fired in one night, some of which contained nearly three gallons of the liquid.
Mustard gas is a high boiling and very persistent material, which is characterized by its vesicant (skin blistering) action. Men who come in contact with it, either in the form of fine splashes of the liquid or in the form of vapor, suffer severe blistering of the skin. The burns appear from four to twelve hours after exposure and heal very slowly. [Pg 23] Ordinary clothing is no protection against either the vapor or the liquid. Other effects will be considered in Chapter IX.
Since then there has been no important advance so far as new gases are concerned. Various arsenic derivatives were prepared in the laboratory and tested on a small scale. The Germans did actually introduce ethyldichloroarsine and the Americans were considering methyldichloroarsine. Attempts were made to improve upon mustard gas but they were not successful.
It is rather a peculiar fact that so few new chemical compounds were used as war gases. Practically all the substances were well known to the organic chemist long before the World War. One of the most interesting and valuable of the compounds which would have found extensive use had the War continued, is an arsenic compound called Lewisite from its discoverer, Capt. W. Lee Lewis, of Northwestern University. The chemistry of this compound is discussed in Chapter X. Because of the early recognized value of this compound, very careful secrecy was maintained as to all details of the method of preparation and its properties. As a result, strange stories were circulated about its deadly powers. Characteristic of these was the story that appeared in the New York Times early in 1919. Now that the English have published the chemical and pharmacological properties, we can say that, although Lewisite was never proven on the battle field, laboratory tests indicate that we have here a very powerful agent. Not only is it a vesicant of about the same order of mustard gas, but the arsenical penetrates the skin of an animal, and three drops, placed on the abdomen of a mouse, are sufficient to kill within two to three hours. It is also a powerful respiratory irritant and causes violent sneezing. Its possible use in aeroplane bombs has led General Fries to apply the term “The Dew of Death” to its use in this way.
Considerable effort was spent on the question of camouflage gases. This involved two lines of research:
(1) To prevent the recognition of a gas when actually present on the field, by masking its odor. [Pg 24]
TABLE I
Chemical Warfare Gases
| Chemical | Belligerent | Effect | Means of Projection |
|---|---|---|---|
| Acrolein (allylaldehyde) | French | Lachrymatory | Hand grenades |
| Lethal | |||
| Arsenic chloride | (In mixtures. See below) | ||
| Benzyl iodide | French | Lachrymatory | Artillery shell |
| Benzyl chloride | French | Lachrymatory | Artillery shell |
| Bromoacetone | French | Lachrymatory | Artillery shell |
| Lethal | |||
| Bromobenzylcyanide | French | Lachrymatory | Artillery shell |
| Bromomethylethylketone | German | Lachrymatory | Artillery shell |
| Lethal | Artillery shell | ||
| Benzyl bromide | German | Lachrymatory | Artillery shell |
| French | |||
| Chlorine | German | Lethal | Cylinders |
| British | (cloud gas) | ||
| French | |||
| American | |||
| Chlorosulfonic acid | German | Irritant | Hand grenades, |
| light minenwerfer | |||
| Chloroacetone | French | Lachrymatory | Artillery shell |
| Chlorobenzene (as solvent) | German | Lachrymatory | Artillery shell |
| Chloropicrin | British | Lethal | Artillery shell |
| French | Lachrymatory | Trench mortar bombs | |
| German | Projectors | ||
| American | [Pg 25] | ||
| Cyanogen bromide | Austrian | Lethal | Artillery shell |
| Dichloromethylether | German | Lachrymatory | Artillery shell |
| (as solvent) | |||
| Diphenylchloroarsine | German | Sternutatory | Artillery shell |
| Lethal | |||
| Dichloroethylsulfide | German | Vesicant | Artillery shell |
| French | Lethal | ||
| British | Irritant | ||
| American | |||
| Ethyldichloroarsine | German | Lethal | Artillery shell |
| Ethyliodoacetate | British | Lachrymatory | Artillery shell, |
| 4-in. Stokes’ mortars, | |||
| hand grenades | |||
| Hydrocyanic acid | French | (In mixtures. See below) | Lachrymatory |
| Methylchlorosulfonate | German | Irritant | Minenwerfer |
| Monochloromethylchloroformate | French | Lachrymatory | Lachrymatory |
| Phosgene | British | Lethal | Projectors, |
| French | trench mortars, | ||
| German | artillery shell, | ||
| American | cylinders | ||
| Phenylcarbylaminechloride | German | Lachrymatory | Artillery shell |
| Irritant | |||
| Trichlormethylchloroformate | German | Lethal | Artillery shell |
| Stannic chloride | British | Irritant | Hand grenades |
| French | Cloud forming | Artillery | |
| American | Projectors | ||
| 4-in. Stokes’ | |||
| mortar bombs | |||
| Sulfuric anhydride | German | Irritant | Hand grenades, |
| minenwerfer, | |||
| artillery shell | |||
| Xylyl bromide | German | Lachrymatory | Artillery shell |
[Pg 26]
TABLE I—Continued
| Chemical | Belligerent | Effect | Means of Projection |
|---|---|---|---|
| Mixtures[4] | |||
| Bromoacetone (80%) and | French | Lachrymatory | Artillery shell |
| Chloroacetone (20%) | Lethal | ||
| Chlorine (50%) and | British | Lethal | Cylinders |
| Phosgene (50%) | German | ||
| Chlorine (70%) and | British | Lethal | Cylinders |
| Chloropicrin (30%) | Lachrymatory | ||
| Chloropicrin (65%) and | British | Lethal | Cylinders |
| Hydrogen sulfide (35%) | Lachrymatory | ||
| Chloropicrin (80%) and | British | Lethal | Artillery shell |
| Stannic chloride (20%) | French | Lachrymatory | Trench mortar bombs |
| American | Irritant | Projectors | |
| Chloropicrin (75%) and | Lethal | Artillery shell | |
| Phosgene (25%) | British | Lachrymatory | Trench mortar bombs, |
| projectors | |||
| Dichloroethyl sulfide (80%) | German | Vesicant | |
| and Chlorobenzene (20%) | French | Lethal | Artillery shell |
| British | |||
| American | |||
| Ethyl carbazol (50%) and | German | Sternutatory | Artillery shell |
| Diphenylcyanoarsine (50%) | Lethal [Pg 27] | ||
| Ethyldichloroarsine (80%) and | German | Lethal | Artillery shell |
| Dichloromethylether (20%) | Lachrymatory | ||
| Ethyliodoacetate (75%) and | Artillery shell, | ||
| Alcohol (25%) | British | Lachrymatory | 4-in. Stokes’ mortars, |
| hand grenades | |||
| Hydrocyanic acid (55%) | British | Lethal | Artillery shell |
| Chloroform (25%) and | |||
| Arsenious chloride (20%) | |||
| Hydrocyanic acid (50%), | |||
| Arsenious chloride (30%), | French | Lethal | Artillery shell |
| Stannic chloride (15%) and | |||
| Chloroform (5%) | |||
| Phosgene (50%) and | British | Lethal | Artillery shell |
| Arsenious chloride (50%) | |||
| Dichloroethyl sulfide (80%) | German | Vesicant | |
| and Carbon tetrachloride (20%) | French | Lethal | Artillery shell |
| British | |||
| American | |||
| Phosgene (60%) and | British | Lethal | Artillery shell |
| Stannic chloride (40%) | French | Irritant | |
| Methyl sulfate (75%) and | French | Lachrymatory | Artillery shell |
| Chloromethyl sulfate (25%) | Irritant |
[Pg 28] (2) To simulate the presence of a toxic gas. This may be done either by using a substance whose odor in the field strongly suggests that of the gas in question, or by so thoroughly associating a totally different odor with a particular “gas” in normal use that, when used alone, it still seems to imply the presence of that gas. This use of imitation gas would thus be of service in economizing the use of actual “gas” or in the preparation of surprise attacks.
While there was some success with this kind of “gas,” very few such attacks were really carried out, and these were in connection with projector attacks.
Table I gives a list of all the gases used by the various armies, the nation which used them, the effect produced and the means of projection used.
Table II gives the properties of the more important war cases (compiled by Major R. E. Wilson, C. W. S.).
The gases used by the Germans may also be classified by the names of the shell in which they were used. Table III gives such a classification.
In selecting markings for American chemical shell, red bands were used to denote persistency, white bands to denote non-persistency and lethal properties, yellow bands to denote smoke, and purple bands to denote incendiary action. The number of bands indicates the relative strength of the property indicated; thus, three red bands denote a gas more persistent than one red band.
The following shell markings were actually used:
| 1 White | Diphenylchloroarsine |
| 2 White | Phosgene |
| 1 White, 1 red | Chloropicrin |
| 1 White, 1 red, 1 white | 75% Chloropicrin, 25% Phosgene |
| 1 White, 1 red, 1 yellow | 80% Chloropicrin, 20% Stannic Chloride |
| 1 Red | Bromoacetone |
| 2 Red | Bromobenzylcyanide |
| 3 Red | Mustard Gas |
| 1 Yellow | White Phosphorus |
| 2 Yellow | Titanium Tetrachloride |
[Pg 29]
TABLE II
Physical Constants of Important War Gases
| Name of Gas | Formula | Molecular Weight |
Liquid Density at 20° C. under Own Pressure |
Melting point, °C. |
Boiling point, °C. |
Vapor Pressure at 20° C. (mm. Hg) |
|---|---|---|---|---|---|---|
| Bromoacetone | C₃H₅BrO | 136.98 | 1.7(?) | - 54 | 126 | 9(?) |
| Carbon monoxide | CO | 28.00 | (Gas) | -207 | -190 | (Gas) |
| Cyanogen bromide | BrCN | 106.02 | 2.01 | 52 | 61.3 | 89 |
| Cyanogen chloride | ClCN | 61.56 | 1.186 | - 6 | 15 | 1002 |
| Chlorine | Cl₂ | 70.92 | 1.408 | -101.5 | 33.6 | 5126 |
| Chloropicrin | Cl₃C(NO₂) | 164.39 | 1.654 | - 69.2 | 112 | 18.9 |
| Dichloroethyl sulfide | (CH₃CHCl₂)S | 169.06 | 1.274 | 12.5 | 216 | .06 |
| Diphenylchloroarsine | (C₆H₅)₂AsCl | 264.56 | 1.422 | 44 | 333 | .0025 |
| Hydrocyanic acid | HCN | 27.11 | .697 | - 14 | 26.1 | 603 |
| Phenyldichloroarsine | C₆H₅AsCl₂ | 210.96 | 1.640 | ... | 253 | .022 |
| Phosgene | COCl₂ | 98.92 | 1.38 | ... | 8.2 | 1215 |
| Stannic chloride | SnCl₄ | 260.54 | 2.226 | - 33 | 114 | 18.58 |
| Superpalite | CCl₃COOCl | 197.85 | 1.65 | ... | 128 | 10.3 |
| Xylyl bromide | ( CH₃)C₆H₄CH₂Br | 185.03 | 1.381 | - 2 | 214.5 | ... |
[Pg 30]
TABLE III
German Shell
| Name of Shell | Shell Filling | Nature of Effect |
|---|---|---|
| B-shell [K₁ shell (White B or BM)] | Bromoketone | Lachrymator |
| (Bromomethylethyl ketone) | ||
| Blue Cross | (a) Diphenylchloroarsine | Sternutator |
| (b) Diphenylcyanoarsine | Sternutator | |
| (c) Diphenylchloroarsine, | ||
| Ethyl carbazol | ||
| C-shell (Green Cross) (White C) | Superpalite | Asphyxiant |
| D-shell (White D) | Phosgene | Lethal |
| Green Cross | (a) Superpalite | Asphyxiant |
| (b) Phenylcarbylaminechloride | ||
| Green Cross 1 | Superpalite 65%, | Asphyxiant |
| Chloropicrin 35% | ||
| Superpalite, | ||
| Green Cross 2 | Phosgene, | Asphyxiant |
| Diphenylchloroarsine | ||
| Green Cross 3 | Ethyldichloroarsine, | |
| (Yellow Cross 1) | Methyldibromoarsine, | Asphyxiant |
| Dichloromethyl ether | ||
| K-shell (Yellow) | Chloromethylchloroformate | Asphyxiant |
| (Palite) | ||
| T-shell (Black or green T) | Xylyl bromide, | Lachrymator |
| Bromo ketone | ||
| Yellow Cross | Mustard gas, | Vesicant |
| Diluent (CCl₄, C₆H₅Cl, C₆H₅NO₂) | ||
| Yellow Cross 1 | See Green Cross 3 |
[Pg 31]
Modern chemical warfare dates from April 22, 1915. Really, however, it may be said to have started somewhat earlier, for Germany undoubtedly had spent several months in perfecting a successful gas cylinder and a method of attack. The Allies, surprised by such a method of warfare, were forced to develop, under pressure, a method of defense, and then, when it was finally decided to retaliate, a method of gas warfare. “Offensive organizations were enrolled in the Engineer Corps of the two armies and trained for the purpose of using poisonous gases; the first operation of this kind was carried out by the British at the battle of Loos in September, 1915.
“Shortly after this the British Army in the field amalgamated all the offensive, defensive, advisory and supply activities connected with gas warfare and formed a ‘Gas Service’ with a Brigadier General as Director. This step was taken almost as a matter of necessity, and because of the continually increasing importance of the use of gas in the war (Auld).”
At once the accumulation of valuable information and experience was started. Later this was very willingly and freely placed at the disposal of American workers. Too much cannot be said about the hearty co-operation of England and France. Without it and the later exchange of information on all matters regarding gas warfare, the progress of gas research in all the allied countries would have been very much retarded.
While many branches of the American Army were engaged in following the progress of the war during 1915-1916, the growing importance of gas warfare was far from being appreciated. When the United States declared war on Germany April 6, 1917, there were a few scattered observations [Pg 32] on gas warfare in various offices of the different branches, but there was no attempt at an organized survey of the field, while absolutely no effort had been made by the War Department to inaugurate research in a field that later had 2,000 men alone in pure research work. Equally important was the fact that no branch of the Service had any idea of the practical methods of gas warfare.
The only man who seemed to have the vision and the courage of his convictions was Van H. Manning, Director of the Bureau of Mines. Since the establishment of the Bureau in 1908 it had maintained a staff of investigators studying poisonous and explosive gases in mines, the use of self-contained breathing apparatus for exploring mines filled with noxious gases, the treatment of men overcome by gas, and similar problems. At a conference of the Director of the Bureau with his Division Chiefs, on February 7, 1917, the matter of national preparedness was discussed, and especially the manner in which the Bureau could be of most immediate assistance with its personnel and equipment. On February 8, the Director wrote C. D. Walcott, Chairman of the Military Committee of the National Research Council, pointing out that the Bureau of Mines could immediately assist the Navy and the Army in developing, for naval or military use, special oxygen breathing apparatus similar to that used in mining. He also stated that the Bureau could be of aid in testing types of gas masks used on the fighting lines, and had available testing galleries at the Pittsburgh experiment station and an experienced staff. Dr. Walcott replied on February 12 that he was bringing the matter to the attention of the Military Committee.
A meeting was arranged between the Bureau and the War College, the latter organization being represented by Brigadier General Kuhn and Major L. P. Williamson. At this conference the War Department enthusiastically accepted the offer of the Bureau of Mines and agreed to support the work in every way possible.
The supervision of the research on gases was offered to Dr. G. A. Burrell, for a number of years in charge of the chemical work done by the Bureau in connection with the investigation of mine gases and [Pg 33] natural gas. He accepted the offer on April 7, 1917. The smoothness with which the work progressed under his direction and the importance of the results obtained were the result of Colonel Burrell’s great tact, his knowledge of every branch of research under investigation and his imagination and general broad-mindedness.
Once, however, that the importance of gas warfare had been brought to the attention of the chemists of the country, the response was very eager and soon many of the best men of the university and industrial plants were associated with Burrell in all the phases of gas research. The staff grew very rapidly and laboratories were started at various points in the East and Middle West.
It was immediately evident that there should be a central laboratory in Washington to co-ordinate the various activities and also to considerably enlarge those activities under the joint direction of the Army, the Navy and the Bureau of Mines. Fortunately a site was available for such a laboratory at the American University, the use of the buildings and grounds having been tendered President Wilson on April 30, 1917. Thus originated the American University Experiment Station, later to become the Research Division of the Chemical Warfare Service.
Meanwhile other organizations were getting under way. The procurement of toxic gases and the filling of shell was assigned to the Trench Warfare Section of the Ordnance Department. In June, 1917, General Crozier, then Chief of the Ordnance Department, approved the general proposition of building a suitable plant for filling shell with toxic gas. In November, 1917, it was decided to establish such a plant at Gunpowder Neck, Maryland. Owing to the inability of the chemical manufacturers to supply the necessary toxic gases, it was further decided, in December, 1917, to erect at the same place such chemical plants as would be necessary to supply these gases. In January, 1918, the name was changed to Edgewood Arsenal, and the project was made a separate Bureau of the Ordnance Department, Col. William H. Walker, of the Massachusetts Institute of Technology, being soon afterwards put in command. [Pg 34]
While, during the latter part of the War, gas shell were handled by the regular artillery, special troops were needed for cylinder attacks, Stokes’ mortars, Livens’ projectors and for other forms of gas warfare. General Pershing early cabled, asking for the organization and training of such troops, and recommended that they be placed, as in the English Army, under the jurisdiction of the Engineer Corps. On August 15, 1917, the General Staff authorized one regiment of Gas and Flame troops, which was designated the “30th Engineers,” and was commanded by Major (later Colonel) E. J. Atkisson. This later became the First Gas Regiment, of the Chemical Warfare Service.
About this time (September, 1917) the need of gas training was recognized by the organization of a Field Training Section, under the direction of the Sanitary Corps, Medical Department. Later it was recognized that neither the Training Section nor the Divisional Gas Officers should be under the Medical Department, and, in January, 1918, the organization was transferred to the Engineer Corps.
All of these, with the exception of the Gas and Flame regiment, were for service on this side. The need for an Overseas force was recognized and definitely stated in a letter, dated August 4, 1917. On September 3, 1917, an order was issued establishing the Gas Service, under the command of Lt. Col. (later Brigadier General) A. A. Fries, as a separate Department of the A. E. F. in France. In spite of a cable on September 26th, in which General Pershing had said
“Send at once chemical laboratory, complete equipment and personnel, including physiological and pathological sections, for extensive investigation of gases and powders....”
it was not until the first of January, 1918, that Colonel R. F. Bacon of the Mellon Institute sailed for France with about fifty men and a complete laboratory equipment.
Meantime a Chemical Service Section had been organized in the United States. This holds the distinction of being the first recognition of chemistry as a separate branch of the military service in any country or any war. This was authorized October 16, 1917, and was to consist of an officer of the Engineers, not above the rank of colonel, who was to be Director of Gas Service, with assistants, not above the rank of [Pg 35] lieutenant colonel from the Ordnance Department, Medical Department and Chemical Service Section. The Section itself was to consist of 47 commissioned and 95 non-commissioned officers and privates. Colonel C. L. Potter, Corps of Engineers, was appointed Director and Professor W. H. Walker was commissioned Lieutenant Colonel and made Assistant Director of the Gas Service and Chief of the Chemical Service Section. This was increased on Feb. 15, 1918 to 227 commissioned and 625 enlisted men, and on May 6, 1918 to 393 commissioned and 920 enlisted men. Meanwhile Lt. Col. Walker had been transferred to the Ordnance and Lt. Col. Bogert had been appointed in his place.
At this time practically every branch of the Army had some connection with Gas Warfare. The Medical Corps directed the Gas Defense production. Offense production was in the hands of the Ordnance Department. Alarm devices, etc., were made by the Signal Corps. The Engineers contributed their 30th Regiment (Gas and Flame) and the Field Training Section. The Research Section was still in charge of the Bureau of Mines, in spite of repeated attempts to militarize it. And in addition, the Chemical Service Section had been formed primarily to deal with overseas work. While the Director of the Gas Service was expected to co-ordinate all these activities, he was given no authority to control policy, research or production.
In order to improve these conditions Major General Wm. L. Sibert, a distinguished Engineer Officer who built the Gatun Locks and Dam of the Panama Canal and who had commanded the First Division in France, was appointed Director of the Chemical Warfare Service on May 11, 1918. Under his direction the Chemical Warfare Service was organized with the following Divisions:
| Overseas | Brigadier General Amos A. Fries |
| Research | Colonel G. A. Burrell |
| Development | Colonel F. M. Dorsey |
| Gas Defense Production | Colonel Bradley Dewey |
| Gas Offense Production | Colonel Wm. H. Walker |
| Medical | Colonel W. J. Lyster |
| Proving | Lt. Col. W. S. Bacon |
| Administration | Brigadier General H. C. Newcomer |
| Gas and Flame | Colonel E. J. Atkisson |
[Pg 36] The final personnel authorized, though never reached owing to the signing of the Armistice, was 4,066 commissioned officers and 44,615 enlisted men; this was including three gas regiments of eighteen companies each.
General Sibert brought with him not only an extended experience in organizing and conducting big business, but a strong sympathy for the work and an appreciation of the problem that the American Army was facing in France. He very quickly welded the great organization of the Chemical Warfare Service into a whole, and saw to it that each department not only carried on its own duties but co-operated with the others in carrying out the larger program, which, had the war continued, would have beaten the German at his own game.
More detailed accounts will now be given of the various Divisions of the Chemical Warfare Service.
The Administration Division was the result of the development which has been sketched in the preceding pages. It is not necessary to review that, but the organization as of October 19, 1918 will be given:
| Director | Major General Wm. L. Sibert |
| Staff: | |
| Medical Officer | Colonel W. J. Lyster |
| Ordnance Officer | Lt. Col. C. B. Thummel |
| British Military Mission | Major J. H. Brightman |
| Assistant Director | Colonel H. C. Newcomer |
| Office Administration | Major W. W. Parker |
| Relations Section | Colonel M. T. Bogert |
| Personnel Section | Major F. E. Breithut |
| Contracts and Patents Section | Captain W. K. Jackson |
| Finance Section | Major C. C. Coombs |
| Requirements and Progress Section | Capt. S. M. Cadwell |
| Confidential Information Section | Major S. P. Mullikin |
| Transportation Section | Captain H. B. Sharkey |
| Training Section | Lt. Col. G. N. Lewis |
| Procurement Section | Lt. Col. W. J. Noonan |
The administrative offices were located in the Medical Department Building. The function of most of the sections is indicated by their names. [Pg 37]
The Industrial Relations Section was created to care for the interests of the industrial plants which were considered as essential war industries. Through its activity many vitally important industries were enabled to retain, on deferred classification or on indefinite furlough, those skilled chemists without which they could not have maintained a maximum output of war munitions.
In the same way the University Relations Section cared for the educational and research institutions. In this way our recruiting stations for chemists were kept in as active operation as war conditions permitted.
Another important achievement of the Administration Section was to secure the order from The Adjutant General, dated May 28, 1918, that read:
“Owing to the needs of the military service for a great many men trained in chemistry, it is considered most important that all enlisted men who are graduate chemists should be assigned to duty where their special knowledge and training can be fully utilized.
“Enlisted men who are graduate chemists will not be sent overseas unless they are to be employed on chemical duties....”
While this undoubtedly created a great deal of feeling among the men who naturally were anxious to see actual fighting in France, it was very important that this order be carried out in order to conserve our chemical strength. The following clipping from the September, 1918, issue of The Journal of Industrial and Engineering Chemistry shows the result of this order.
“Chemists in Camp
“As the result of the letter from The Adjutant General of the Army, dated May 28, 1918, 1,749 chemists have been reported on. Of these the report of action to August 1, 1918, shows that 281 were ordered to remain with their military organization because they were already performing chemical duties, 34 were requested to remain with their military organization because they were more useful in the military work which they were doing, 12 were furloughed back to industry, 165 [Pg 38] were not chemists in the true sense of the word and were, therefore, ordered back to the line, and 1,294 now placed in actual chemical work. There were being held for further investigation of their qualifications on August 1, 1918, 432 men. The remaining 23 men were unavailable for transfer, because they had already received their overseas orders.
“The 1,294 men, who would otherwise be serving in a purely military capacity and whose chemical training is now being utilized in chemical work, have, therefore, been saved from waste.
“Each case has been considered individually, the man’s qualifications and experience have been studied with care, the needs of the Government plants and bureaus have been considered with equal care, and each man has been assigned to the position for which his training and qualifications seem to fit him best.
“Undoubtedly, there have been some cases in which square pegs have been fitted into round holes, but, on the whole, it is felt that the adjustments have been as well as could be expected under the circumstances.”
The American University Experiment Station, established by the Bureau of Mines in April, 1917, became July 1, 1918 the Research Division of the Chemical Warfare Service. For the first five months work was carried out in various laboratories, scattered over the country. In September, 1917, the buildings of the American University became available; a little later portions of the new chemical laboratory of the Catholic University, Washington, were taken over. Branch laboratories were established in many of the laboratories of the Universities and industrial plants, of which Johns Hopkins, Princeton, Yale, Ohio State, Massachusetts Institute of Technology, Harvard, Michigan, Columbia, Cornell, Wisconsin, Clark, Bryn Mawr, Nela Park and the National Carbon Company were active all through the war.
At the time of the signing of the armistice the organization of the Research Division was as follows: [Pg 39]
| Col. G. A. Burrell | Chief of Research Division |
| Dr. W. K. Lewis | In Charge of Defense Problems |
| Dr. E. P. Kohler[5] | In Charge of Offense Problems |
| Dr. Reid Hunt | Advisor on Pharmacological Problems |
| Lt. Col. W. D. Bancroft | In Charge of Editorial Work and Catalytic Research |
| Lt. Col. A. B. Lamb[6] | In Charge of Defense Chemical Research |
| Dr. L. W. Jones[7] | In Charge of Offense Chemical Research |
| Major A. C. Fieldner | In Charge of Gas Mask Research |
| Major G. A. Richter | In Charge of Pyrotechnic Research |
| Capt. E. K. Marshall[8] | In Charge of Pharmacological Research |
| Dr. A. S. Loevenhart[9] | In Charge of Toxicological Research |
| Major R. C. Tolman | In Charge of Dispersoid Research |
| Major W. S. Rowland[10] | In Charge of Small Scale Manufacture |
| Major B. B. Fogler[11] | In Charge of Mechanical Research and Development |
| Captain G. A. Rankin | In Charge of Explosive Research |
| Major Richmond Levering | In Charge of Administration Section |
The chief functions of the Research Division were: [Pg 40]
1. To prepare and test compounds which might be of value in gas warfare, determining the properties of these substances and the conditions under which they might be effective in warfare.
2. To develop satisfactory methods of making such compounds as seemed promising (Small Scale).
3. To develop the best methods of utilizing these compounds.
4. To develop materials which should absorb or destroy war gases, studying their properties and determining the conditions under which they might be effective.
5. To develop satisfactory methods of making such absorbents as might seem promising.
6. To develop masks, canisters, protective clothing, etc.
7. To develop incendiaries, smokes, signals, etc., and the best methods of using the same.

Fig. 4.—American University Experiment Station,
showing Small Scale Plants.
8. To co-operate with the manufacturing divisions in regard to difficulties arising during the operations of manufacturing war gases, absorbents, etc.
9. To co-operate with other branches of the Government, civil and military, in regard to war problems.
10. To collect and make available to the Director of the Chemical Warfare Service all information in regard to the chemistry of gas warfare.
The relation of the various sections may best be shown by outlining the general procedure used when a new toxic substance was developed. [Pg 41]
The substance in question may have been used by the Germans or the Allies; it may have been suggested by someone outside the station; or the staff may have thought of it from a search of the literature, from analogy or from pure inspiration. The Offense Research Section made the substance. If it was a solid it was sent to the Dispersoid Section, where methods of dispersing it were worked out. When this had been done, or, at once, if the compound was a liquid or vapor, it was sent to the Toxicological Section to be tested for toxicity, lachrymatory power, vesicant action, or other special properties. If these tests proved the compound to have a high toxicity or a peculiar physiological behavior, it was then turned over to a number of different sections.
The Offense Research Section tried to improve the method of preparation. When a satisfactory method had been found, the Chemical Production or Small Scale Manufacturing Section endeavored to make it on a large scale (50 pounds to a ton) and worked out the manufacturing difficulties. If further tests showed that the substance was valuable, the manufacture was then given to the Development Division or the Gas Offense Production Division for large scale production.
Meanwhile the Analytical Section had been working on a method for testing the purity of the material and for analyzing air mixtures, and the Gas Mask Section had run tests against it with the standard canisters. If the protection afforded did not seem sufficient, the Defense Chemical Section studied changes in the ingredients of the canister or even developed a new absorbent or mixture of absorbents to meet the emergency. If a change in the mechanical construction of the canister was necessary, this was referred to the Mechanical Research Section; this work was especially important in case the material was to be used as a toxic smoke.
The compound was also sent to the Pyrotechnic Section, which studied its behavior when fired from a shell, or, if suitable, when used in a cylinder. If it proved stable on detonation, large field tests were then made by the Proving Division, in connection with the Pyrotechnic and Toxicological Sections of the Research Division, to learn the effect when shell loaded with the compound were fired from guns on a [Pg 42] range, with animals placed suitably in or near the trenches. The Analytical Section worked out methods of detecting the gas in the field, wherever possible.
The Medical Division, working with the Toxicological and Pharmacological Sections, studied pathological details, methods of treating gassed cases, the effect of the gas on the body, and in some cases even considered other questions, such as the susceptibility of different men.
If the question of an ointment or clothing entered into the matter of protection, these were usually attacked by several Sections from different points of view.
Out of the 250 gases prepared by the Offense Chemical Research Section, very few were sufficiently valuable to pass all of these tests and thus the number of gases actually put into large scale production were less than a dozen. This had its advantages, for it made unnecessary a large number of factories and the training of men in the manufacturing details of many gases. As one British report stated, “The ultimate object of chemical warfare should be to produce two substances only; one persistent and the other non-persistent; both should be lethal and both should be penetrants.” They might well have added that both should be instantly and powerfully lachrymatory.
Since most of the work of the Research Division will be covered in detail in later chapters, only a brief summary of the principal problems will be given here.
The first and most important problem was the development of a gas mask. This was before Sections had been organized and was the work of the entire Division. After comparing the existing types of masks it was decided that the Standard Box Respirator of the British was the best one to copy. Because we were entirely new at the game that meant work on charcoal, soda-lime, and the various mechanical parts of the mask, such as the facepiece, elastics, eyepieces, mouthpiece, noseclip, hose, can, valves, etc. The story of the “first twenty thousand” is very well told by Colonel Burrell.[12]
[Pg 43]
“The First Twenty Thousand
“About the first of May, 1917, Major L. P. Williamson, acting as liaison officer between the Bureau of Mines and the War Department, put the last ounce of ‘pep’ into the organization by asking us to build 20,000 gas masks for shipment overseas. 20,000 masks did not seem like a very large order. We did not fully appreciate all the conditions which a war gas mask had to encounter, so we readily and willingly accepted the order. Then began a struggle with can manufacturers, buckle makers, manufacturers of straps, rubber facepieces, eyepieces, knapsacks, etc. The country was canvassed from the Atlantic Coast to the Mississippi River for manufacturers who could turn out the different parts acceptably and in a hurry.
“Charcoal was made from red cedar by the Day Chemical Co. of Westline, Pennsylvania; soda-lime permanganate was manufactured by the General Chemical Company; knapsacks by the Simmons Hardware Company in St. Louis; facepieces by the Goodrich and Goodyear Rubber Companies at Akron; canisters by the American Can Company; and the assembly made at one of the plants of the American Can Company in Long Island City.
“The writer cannot recall all the doubts, fears, optimism, and enthusiasm felt in turn by different members of the organization during the fabrication of those first 20,000 masks. We were performing an important task for the War Department. Night became day. Dewey, Lewis, Henderson, Gibbs, and others stepped from one train to another, and we used the telephone between Washington and St. Louis or Boston as freely as we used the local Washington telephone.
“We thought we could improve on the English box respirator on various points. We made the canister larger, and have been glad ever since that we did. We thought the English mouthpiece was too flexible and too small, and made ours stiff and larger, and were sorry we made the change. We tested the fillings against chlorine, phosgene, prussic acid, etc., and had a canister that was all that was desired for absorbing these gases. But, alas, we did not know that chloropicrin was destined to be one of the most important war gases used by the various belligerents. Further, it was not fully appreciated that the rubberized cloth used in making the facepiece had to be highly impermeable against gases, that hardness as much as anything else was desired in the make-up of the soda-lime granules in order to withstand rough jolting so that the fines would not clog the canister, and raise the resistance to breathing to a prohibitive figure. Neither was it appreciated at [Pg 44] that time by any of the allies, that the gas mask really should be a be a fighting instrument, one that men could work hard in, run in, and wear for hours, without too serious discomfort.
“The first 20,000 masks sent over to England were completed by the Research Division in record time. As compared with the French masks, they were far superior, giving greater protection against chlorine, phosgene, superpalite, prussic acid, xylyl bromide, etc. The French mask was of the cloth type, conforming to the face, and consisting of twenty layers of cheesecloth impregnated with sodium phenate and hexamethylenetetramine. Chloropicrin went through this like a shot. Just before the masks were sent abroad, we received disturbing rumors of the contemplated use of large quantities of chloropicrin. The French, apparently, had no intention of changing the design of their mask, and did not do so for months to come. We therefore released the masks, they were sent abroad, and an anxious research group on this side of the water waited expectantly for the verdict. It came. A brief cablegram told us what our English cousins thought of us. It was a subject they had been wrestling with for two years and a half. They had had battlefield experience; they had gone through the grief of developing poor masks into better ones, knew the story better than we did, and after a thorough test ‘hammered’ the American design unmercifully.
“This experience put the Research Division on its mettle. Our first attempt had given us the necessary preliminary experience; cablegrams and reports traveled back and forth; an expert or two eventually came to this country from England in response to previous appeals for assistance, and we turned with adequate information to the development of a real mask.”
The story of mustard gas is given later. It probably occupied more time and thought on the part of the Research Division, as well as that of Edgewood Arsenal and the Development Division, than any other gas.
Diphenylchloroarsine led to the preparation of a series of arsenic compounds, some more easily prepared and more or less effective.
Cyanogen chloride and cyanogen bromide, reported by the Italians as having been used by the Germans, were extensively studied.
The Inorganic Section was early interested in special incendiary materials which were developed for bombs, shells, darts and grenades, [Pg 45] and which were later taken over by the Pyrotechnic Section, and finally adopted by the Ordnance Department.
In discussing the work one can very well start with the Offense Section. This Section had two aims in view always, to develop methods of making the gases used by the Germans more economically than they were making them, and to develop better gases if possible. When we entered the war, chlorine, phosgene and chloropicrin were the lethal gases used, while bromoacetone and xylyl bromide were the lachrymators. It was not a difficult matter to prepare these. But the introduction of mustard gas in the summer of 1917 and of diphenylchloroarsine in the autumn of the same year, not only made our chemists ponder over a manufacturing method, but also so revised our notions of warfare that the possibility of using other substances created the need for extensive research. The development of bromobenzylcyanide by the French likewise opened a new field among lachrymatory substances.
Colored rockets and smokes were developed for the Navy and Army. The smoke box was also studied but the work was taken over by the Pyrotechnic Section.
A large amount of pure inorganic research on arsine and arsenides, fluorine, hydrofluoric acid and fluorides, cyanides, cyanogen sulfide and nitrogen tetroxide was carried out, sometimes successfully and at other times with little or no success.
The Analytical Section not only carried out all routine analyses but developed methods for many new gases.
The Offense Section worked in very close contact with the Small Scale Manufacturing Section (Chemical Production Section). Often it happened that a method, apparently successful in the laboratory, was of no value in the plant. Small scale plants were developed for mustard gas, hydrocyanic acid, cyanogen chloride, arsenic trichloride, arsenic trifluoride, magnesium arsenide, superpalite and bromobenzylcyanide.
The Chemical Defense Section, organized January, 1918, was occupied with problems relating to protection, such as charcoal, soda-lime, and special absorbents, eyepieces, smoke filters, efficiency of absorbents, and special work with mustard gas. [Pg 46]
Charcoal demanded extensive research. Raw materials required a world-wide search, carbonizing methods had to be developed, and impregnating agents were thoroughly studied. This story is told in Chapter XIII.
Soda-lime was likewise a difficult problem. Starting with the British formula, the influence of the various factors was studied and a balance between a number of desirable qualities, absorptive activity, capacity, hardness, resistance to abrasion, chemical stability, etc., obtained. The final product consisted of a mixture of lime, cement, kieselguhr, sodium permanganate and sodium hydroxide.
Equally valuable work was performed in the perfection of two carbon monoxide absorbents for the Navy. The better of these consisted of a mixture of suitably prepared oxides which acts catalytically under certain conditions, and causes the carbon monoxide to react with the oxygen of the air. Since there are color changes connected with the iodine pentoxide reaction (the first absorbent) it has been possible to develop this so as to serve as a very sensitive detector for the presence of carbon monoxide in air.
While the question of smoke filters was so important that it occupied the attention of several Sections, the Defense Section developed, as a part of its work, a standard method of testing and comparing filters, and did a great deal of work on the preparation of paper for this purpose.
Various problems related to mustard gas were also studied. The question of a protective ointment was solved as successfully as possible under the circumstances, but was dropped when it appeared doubtful if under battlefield conditions of concentration and length of exposure, any ointment offered sufficient protection to pay for the trouble of applying it. The removal of mustard gas from clothing was investigated, especially by the accelerating effect of turkey red oil. Another phase of the work concerned the destruction of mustard gas on the ground, while a fourth phase related to the persistency of mustard (and other gases) on the field of battle.
The Gas Mask Research Section concerned itself largely with developing methods of testing canisters and with routine tests. When one considers [Pg 47] the number of gases studied experimentally, the large number of experimental canisters developed, all of which were tested against two or more gases, and further that the Section assisted in the control of the production at Long Island City, it is seen that this was no small job. In addition, the effect of various conditions, such as temperature, humidity, ageing, size of particles, were studied in their relation to the life of absorbents and canisters. Man tests and mechanical tests will be discussed in a later chapter. Other studies were concerned with weathering tests of gas mask fabrics, mustard gas detector, and covering for dugout entrances (dugout blankets), which were impregnated with a mixture of mineral and vegetable oils. In studying the course of gases through a canister the “wave front” method was of great value in detecting defects in canister design and filling.
The Pyrotechnic Section was composed of a number of units, each with its own problem. The gas shell was studied, with special reference to the stability of gases and toxic solids, both on storage and on detonation. Extensive work was carried out on smoke screens—a Navy funnel, an Army portable smoke apparatus, using silicon tetrachloride, a grenade, a Livens, and various shell being developed for that purpose. The smoke screen was adapted to the tank and the airplane as well as to the funnel of a ship. Several types of incendiary bombs and darts were perfected. The liquid fire gun was studied but the results were never utilized because of the abandonment as useless of that form of warfare. Various forms of signal lights, flares, rockets and colored smokes were studied and in most cases specifications were written. Extensive studies were also carried out on gas shell linings, from which a lead and an enamel lining were evolved. Many physical properties of war gases and their mixtures were determined.
The Dispersoid Section studied the production of smokes or mists from various solid and liquid substances. Apparatus were developed to study the concentration of smoke clouds and their rate of settling. The efficiency of various filters and canisters was determined, and among other things, a new smoke candle was perfected. [Pg 48]
Mechanical research at first was related to design and construction of a canister and mask, based on the English type. During the latter part of 1917 the Tissot type of mask was studied and then turned over to the Gas Defense Division. A Navy Head Mask and canister was perfected. The horse mask was developed along the lines of the British type, and also a dog mask of the same general nature. Horse boots were also constructed, though they never were used at the front. Many Ordnance and Pyrotechnic problems were also successfully completed, not the least of which was a noiseless gas cylinder. This section developed the first special poison gas suit, composed of an oilcloth suit, a mask and helmet and a special canister.
The Manufacturing Development Section had general charge of the defense problems, and really acted as an emergency section, filling in as occasion demanded. They developed mustard gas clothing and a horse mask. They constructed a hydrogen plant at Langley Field, assisted in solving the difficulties relating to Batchite charcoal at Springfield, Mass., and co-operated in the study of paper and felt as filtering materials for smokes. Towards the close of the war the Section was interested in the application of the gas mask to the industries.
The Physiological work is discussed under the Medical Division.
The Editorial Section received reports from all the other Sections, from which a semi-monthly report was written, and distributed to authorized representatives of the Army and Navy and to our Allies. Reports were also received from abroad and the information thus received was made available to the Research Division. As the number of reports increased the work was collected together into monographs on the various war gases, absorbents, smokes, etc. After the signing of the armistice these were revised and increased in number, so that about fifty were finally turned over to the Director of the Chemical Warfare Service.
The story of the Gas Defense Division is largely the story of the gas mask. Colonel (then Mr.) Bradley Dewey was in charge of the “first [Pg 49] twenty thousand.” Soon after that work was undertaken, he was commissioned Major in the Gas Defense Division of the Sanitary Corps and was placed in charge of the entire manufacturing program. The work of the Division included the development and manufacture as well as the testing and inspection of gas masks, and other defense equipment. The magnitude of the work is seen from the following record of production: 5,692,000 completed gas masks, 3,614,925 of which were produced at the Long Island City Plant, while the remainder were assembled at the Hero Manufacturing Company’s Plant at Philadelphia, 377,881 horse masks, 191,388 dugout blankets, 2,450 protective suits and 1,773 pairs of gloves, 1,246 tons of protective ointment, 45,906 gas warning signals (largely hand horns), 50,549 trench fans and many oxygen inhalators.

Fig. 5.—The Defective Gas Mask.
Successfully used by the Gas Defense Division to stimulate care in every part of the operation of the manufacture of Gas Masks.]
The story of the “first twenty thousand” has already been told on page 43. [Pg 50] That these masks were far from satisfactory is no reflection upon the men who made them. Even with the standard design of the British as a pattern, it was impossible to attain all the knowledge concerning gas masks in two months. The experience gained in this struggle enabled the Army to take up the manufacture of gas masks, in July, 1917, with a more complete realization of the seriousness of the task. The masks were not lost, either, for they were sent to the various camps as training masks and served a very useful purpose.
The first order after this was for 1,100,000 masks, to be completed within a year from date. For this production there was authorized one major, two captains, and ten lieutenants. How little the problem was understood is evident when we realize that in the end there were 12,000 employees in the Gas Defense Plant at Long Island City, N. Y. The first attempts were to secure these through existing concerns. The Hero Manufacturing Company of Philadelphia undertook the work and carried on certain portions of it all through the War. Experience soon showed, however, that because of the necessity for extreme care in the manufacture and inspection of the mask, the ordinary commercial organization was not adapted to carry on their manufacture on the scale necessitated by the Army program. Consequently, on Nov. 21, 1917, the Secretary of War authorized the establishment of a government operated plant, and experienced officials were drawn from New York, Chicago, Boston and other manufacturing centers to carry on the work. Buildings in Long Island City, not far from the chemical plant (charcoal and soda lime) at Astoria, were taken over by the officers of the Gas Defense Service, until in July, 1918, five large buildings were occupied, having a total floor space of 1,000,000 square feet (23 acres). The organization grew from the original thirteen officers until it included some 12,000 employees of whom about 8,500 were women. Because of the care required in all the work, attempt was made to secure, as far as possible, those who had relatives with the A. E. F. The thought was that their personal interest in the work would result in greater care in manufacture and inspection. The personnel was unique in that the [Pg 51] authority was apparently divided between civilian and military, but there was no friction because of this. The efficiency of the entire organization is shown by the fact that the masks manufactured at Long Island City cost fifty cents less per mask than those manufactured under contract.
The first actual shipment (overseas) of box respirators was made from the Gas Defense Plant on March 4, 1918. From this date the production increased by leaps and bounds. As mentioned above, between this date and November 26, when the last mask was manufactured, 3,146,413 masks of the box respirator type were passed through final inspection in the plant. The greatest daily production, 43,926 masks, was reached on October 26, 1918. The process of manufacture will be discussed under the chapter on the Gas Mask.
During the last half of 1918 the Kops Tissot mask was manufactured. This mask had been perfected during the months preceding August, 1918, when its manufacture was started. Considerable difficulty was encountered in its production, but the first mask was completed on September 14, and between that time and the Armistice, 189,603 masks of this type had been manufactured.
Along with this manufacturing development went the building up of an elaborate procurement force charged with the responsibility of providing parts to be assembled at the Gas Defense Plant and at the Hero Manufacturing Company. This Section faced a hard and intricate task, but, though there were instances where the shortage of parts temporarily caused a slowing down of production, these were remarkably rare. Not only had the parts to be standardized, and specifications written, but a field inspection force had to be trained in order that the finished parts might be suitable for the final assembly plant. The problem was further complicated by the fact that the design was constantly changing, as improvement followed improvement. Officers, trained in inspection in a day, were sent out to train inspectors in the industrial centers.
In February, 1918, shortly before the German drive commenced, requisitions were received for sample lots of oiled mittens and oiled [Pg 52] union suits as protection against mustard gas. These were prepared in quantity and sent to the front, as was also a considerable amount of chloride of lime for neutralizing the mustard gas in the field.
Another phase of the work consisted of the Field Testing Section, which was organized to provide field testing conditions for the regular product and for the development organization. Later there were added a preliminary course of training for officers for overseas duty in chemical warfare, the military training of the Gas Defense officers located in and near New York and the training of boat crews engaged in carrying offensive gas supplies. The Field Testing Section rendered valuable service in pointing out weaknesses of designs as developments took place and especially those uncomfortable features of the masks which were apparent only through long wear. During the course of this work the section built a complete trench system in the Pennsylvania Railroad yards with an elaborate dugout, the equal of any of the famous German quarters on the Western front.
The chapters on Charcoal, Soda-Lime and the Gas Mask must be read in this connection to gain an idea of the work carried out by this Division. It is summed up in the statement that American soldiers were provided with equipment which neutralized the best effects of German chemical knowledge as evidenced by the offensive methods and materials employed.
The organization of the Gas Defense Division, as of Nov. 11, 1918, was as follows:
| Colonel Bradley Dewey | Officer in Charge |
| Lieut. Col. A. L. Besse | Asst. Officer in Charge |
| Major M. L. Emerson | Administration Section |
| Major H. P. Schuit | Comptrolling Section |
| Mr. R. Skemp | Procurement Section |
| Major C. R. Johnson | Technical Director |
| Capt. K. Atterbury | Field Testing Section |
| Major J. C. Woodruff | Chemical Manufacturing and |
| Development | |
| Mr. R. R. Richardson | Manager, Gas Defense Plant |
| Capt. H. P. Scott | Officer in Charge, |
| Hero Manufacturing Co. | |
| Major L. W. Cottman | Engineering Branch |
| Major T. L. Wheeler | Chemical Development |
| Major I. W. Wilson | Astoria Branch |
| Capt. W. E. Brophy | San Francisco Branch |
| Lt. E. J. Noble | Cleveland Branch |
| Lt. L. Merrill | Springfield Branch |
[Pg 53]
The Ordnance Department, in making plans for a shell filling plant, thought to interest existing chemical firms in the manufacture of the required toxic materials. As plans developed, however, difficulties arose in carrying out this program. The manufacture of such material at private plants necessitated its shipment to the filling plant at Edgewood. The transportation of large quantities of highly toxic gases seemed attended with great danger. The Director General of Railroads ruled that all such shipments must be made by special train, a very expensive method of transportation. Still more serious objections were encountered in the attempt to enlist the co-operation of existing firms. They recognized that the manufacture of such material would be attended by very great danger; that the work would be limited to the duration of the war; and that the processes involved, as well as the plants necessary for carrying out their processes, would have little post-war value. Moreover, such firms as had the personnel and equipment were already over-worked. With a few exceptions (notably the American Synthetic Color Company, the Oldbury Electro-Chemical Co., Zinsser & Co., and the Dow Chemical Company) they were unwilling to undertake work of this character on any terms whatever.
Early in December, 1917, therefore, it was decided to erect, on the site of the shell filling plant, such chemical plants as would be necessary to furnish the toxic materials required for filling the shell. The Arsenal is situated in an isolated district, twenty miles east of Baltimore, Maryland, on the Pennsylvania Railroad, and comprises 3,400 acres. Since the main line of the Pennsylvania Railroad runs on one side of the tract, while on another is the Bush River, only a few miles from its mouth in Chesapeake Bay, the tract was ideally situated for shipping. This site was referred to, at first, as “Gunpowder Reservation,” but on May 4, 1918, the name was officially changed to “Edgewood Arsenal.” [Pg 54]

Fig. 6.—Edgewood Arsenal.
The upper view shows the site as it appeared Oct. 24, 1917. The lower view shows the same as it appeared nine months later.
[Pg 55] Some idea of the extent of the work may be gained from the following facts. On October 1, 1918, there were 233 officers, 6,948 enlisted men and 3,066 civilians engaged in work at Edgewood. 86 cantonments were built, accommodating about 8,500 men, while the five officers’ barracks provided accommodations for 290. The completed hospital unit consisted of 34 buildings, accommodating 420 patients under ordinary conditions. The total number of buildings erected on the Arsenal grounds was 550. 14.8 miles of improved roads were built, and 21 miles of standard gauge and 15 miles of narrow gauge railway. A system furnishing 9.5 million gallons of salt water and another furnishing two millions of fresh water daily were successfully installed. Large power plants were built in connection with the shell filling plants and the chlorine plant.
Plants for phosgene, chloropicrin, mustard gas, chlorine and sulfur chloride were built and placed in successful operation. Most of the raw materials, with the exception of sulfur chloride, were obtained from commercial firms. The other gases and manufactured materials used, such as phosphorus, tin and silicon tetrachlorides, bromobenzylcyanide and arsenic derivatives were supplied by various plants scattered through the East and Middle West States.
The raw materials used by the Arsenal in 1918 were as follows:
| Salt | 17,358,000 | pounds |
| Bleach | 42,384,000 | “ |
| Picric acid | 3,718,000 | “ |
| Alcohol | 3,718,000 | “ |
| Sulfur | 24,912,000 | “ |
| Sulfur chloride | 6,624,000 | “ |
| Bromine | 238,000 | “ |
| Benzyl chloride | 26,000 | “ |
The production of toxic materials and the amount shipped overseas in bulk follow: [Pg 56]
| Production, Pounds |
Shipped in Bulk, Pounds |
|
|---|---|---|
| Chlorine: | ||
| Liquid | 5,446,000 | 2,976,000 |
| Gaseous | 2,208,000 | |
| Chloropicrin | 5,552,000 | 3,806,000 |
| Phosgene | 3,233,070 | 840,000 |
| Mustard gas | 1,422,000 | 380,000 |
| Bromobenzyl cyanide | 10,000 | |
| White phosphorus | 2,012,000 | 342,000 |
| Tin tetrachloride | 2,012,000 | 212,000 |
| Titanium tetrachloride | 362,000 |
For nearly a month previous to the signing of the Armistice, the various plants at the Arsenal had shut down or were operated only to an extent sufficient to maintain the machinery and equipment in good working order, on account of the lack of shell into which to fill the gas, so that the above figures do not at all represent maximum productive capacity.
These plants will be described in the appropriate chapters.
The shell filling plant was really composed of several small plants, each of which was made up of units radiating from a central refrigeration plant which would serve all the units. Each unit could then be fitted with machinery adapted for filling shell of a different size, and for a particular gas. Moreover, an accident in one of the units would in no way impair the working of the remainder.
The problem involved in the filling of a shell with toxic material (which is always a liquid or a solid and never a gas under the conditions in which it is loaded in the shell) is similar in a way to that of filling bottles with carbonated water. In the development of plans for the filling plant, many suggestions were obtained from a study of the apparatus used in commercial bottling plants. It was necessary to keep in mind not only the large number of shell to be filled, but also the highly toxic character of the filling material to be used. It was essential that the work of filling and closing the [Pg 57] shell should be done by machinery in so far as that was possible, and that the operation should be carried out in a thoroughly ventilated room or tunnel, arranged so that the machinery contained in the tunnel could be operated from the outside. Special care was taken in closing the shell, the closing being accomplished by motors actuated by compressed air, which, in the closing process were driven until they stalled. In this way a uniform closing torque was obtained. The final results secured were admirable, as is evidenced by the fact, reported by the Quartermaster Officer at Vincennes on November 15, 1918, that not a single leaky shell had been found among the 200,000 shell received up to that date.
Fig. 7.—A Typical Shell filling Plant at Edgewood Arsenal.
Details of the filling process will be found in the chapter on Phosgene.
Besides the ordinary gas filling plants (of which one was completed and [Pg 58] two were 80 per cent completed) there was a plant for stannic chloride grenades, one for white phosphorus grenades, and one for smoke shell also filled with phosphorus and a plant for filling incendiary bombs.
Shell are designated by their diameter in inches or millimeters. The approximate amount of toxic gas required for filling each type of shell (10.5 per cent void) is as follows:
| Shell | Phosgene, Pounds |
N. C.,[13] Pounds |
Mustard Gas, Pounds |
|---|---|---|---|
| 75 mm | 1.32 | 1.75 | 1.35 |
| 4.7 inch | 4.27 | 6.20 | 4.20 |
| 155 mm | 11.00 | 15.40 | 10.35 |
| 8 inch | 22.00 | 30.30 | 21.60 |
| Livens | 30.00 |
The gas grenades held 0.446 pound of stannic chloride, and the smoke grenades held 0.67 pound of white phosphorus.
The only type of shell filled was the 75 mm. variety, because either the shell of the other sizes or the accompanying boosters (bursting charges) were not available.
The work done by the filling plant is shown by the following figures, representing the number of shell, grenades, etc.
75 mm. Shell
| Filled | Shipped Overseas |
|
|---|---|---|
| Phosgene | 2,009 | |
| N. C. | 427,771 | 300,000 |
| Mustard gas | 155,025 | 150,000 |
| Livens Drum |
||
| Phosgene | 25,689 | 18,600 [Pg 59] |
| Grenades |
||
| White phosphorus | 440,153 | 224,984 |
| Tin tetrachloride | 363,776 | 175,080 |
| Incendiary Drop Bomb |
||
| Mark I. | 542 | |
| Mark II. | 2,104 | |
The total monthly capacity of the filling plants at the date of the Armistice was as follows:
| Pounds | |
| 75 mm. shell | 2,400,000 |
| 4.7 inch shell | 450,000 |
| 155 mm. shell | 540,000 |
| 6 inch shell | 180,000 |
| Gas grenade | 750,000 |
| Smoke grenade | 480,000 |
| Livens drum | 30,000 |
One point relating to the casualties resulting from the work should perhaps be mentioned here. The number of casualties should change the mind of anyone who feels that men chose this work as being “safe” instead of going to France. During the six months from June to December there were 925 casualties, of which three were fatal, two being due to phosgene and one to mustard gas. These were divided among the different gases as follows:
| Mustard gas | 674 |
| Stannic chloride | 50 |
| Phosgene | 50 |
| Chloropicrin | 44 |
| Chlorine | 62 |
| Other material | 45 |
Of these 279 occurred during August, 197 during September and 293 during October. Since production stopped early in November, there were only 14 during that month and three during December. [Pg 60]
The Staff at Edgewood Arsenal at the signing of the Armistice was as follows:
| Commanding Officer | Colonel Wm. H. Walker | |
| Administrative Officers | |
Lt. Colonel George Cahoon, Jr. |
| Lt. Col. Edward M. Ellicott | ||
| Lt. Col. Wm. C. Gallowhur | ||
| In Charge of Outside Plants | |
Lt. Col. Wm. McPherson |
| Major Adrian Nagelvoort | ||
| Major Charles R. Wraith | ||
| Captain John D. Rue | ||
| Shell Filling Plant | Lt. Col. Edwin M. Chance | |
| Chlorine Plant | Lt. Col. Charles Vaughn | |
| Chemical Plants | Major Dana J. Demorest | |
| Chemical Laboratory | Major William L. Evans | |
As the work of the Arsenal expanded it was necessary to manufacture certain of the chemicals at outside plants. The men in charge of these plants were:
| Bound Brook, N. J. | Lt. William R. Chappell |
| Stamford, Conn. | Lt. V. E. Fishburn |
| Hastings-on-Hudson, N. Y. | Major F. G. Zinnsser |
| Niagara Falls, N. Y. | Major A. Nagelvoort |
| Buffalo, N. Y. | Lt. A. W. Davison |
| Kingsport, Tenn. | Lt. E. M. Hayden |
| Charleston, W. Va. | Lt. M. R. Hoyt |
| Midland, Mich. | Major M. G. Donk |
| Croyland, Pa. | Capt. A. S. Hulburt |
After the Armistice, Edgewood Arsenal was selected as the logical home of the Chemical Warfare Service, and all the outside activities of the Service were gradually closed up and the physical property and files moved to Edgewood. At first the command of the Arsenal was in the hands of Lt. Col. Fries, but when he was appointed Chief of the Service, Major E. J. Atkisson, who had so successfully commanded the First Gas Regiment, A. E. F., was happily chosen his successor. At the present time (July 1, 1921), the organization of Edgewood Arsenal is as follows: [Pg 61]
| Commanding Officer | Major E. J. Atkisson |
| Executive Officer | Major R. C. Ditto |
| Technical Director | Dr. J. E. Mills |
| Chemical Division | Mr. D. B. Bradner |
| Mechanical Division | Mr. S. P. Johnson |
| Plant Division | Capt. E. G. Thompson |
| Chemical Warfare School | Major O. R. Meredith |
| Property | Major A. M. Heritage |
| First Gas Regiment | Major C. W. Mason |
| Mask Production Division | Lt. L. A. Elliott |
| Medical Department | Major T. L. Gore |
| Pathological Division | Lt. H. A. Kuhn |
| Quartermaster Department | Capt. H. L. Hudson |
| Finance Department | Capt. C. R. Insley |
The Development Division had its origin in the research laboratories of the National Carbon Company and of the National Lamp Works of the General Electric Company. Both of these companies knew charcoal, and they were asked to produce a satisfactory absorbent charcoal. The success of this undertaking will be seen in the chapter on Absorbents. After a short time all the laboratory work was taken over by the National Carbon Co., while the developmental work was assigned to the National Lamp Works. When the final organization of the Chemical Warfare Service took place, the National Carbon Laboratory became part of the Research Division, while the National Lamp Works became the Defense Section of the Development Division.
The Development Division may be considered as having been composed of the following sections:
The work of the Defense Section consisted of the development of a charcoal suitable for use in gas masks, and its manufacture. While the details will be given later, it may be mentioned here that three weeks after the organization of the Section (April 28, 1917) the furnaces of the National Carbon Company were turning out cedar charcoal, using a straight distillation procedure. Cedar was selected from a large variety of materials as giving the highest absorptive value against chlorine. But phosgene and chloropicrin were also being used, and it was [Pg 62] found that the cedar charcoal was not effective against either. Proceeding on a definite hypothesis, fifty materials were investigated to find the charcoal with the highest density. Cocoanut hulls furnished the raw material, which yielded the most active charcoal. By a process of air activation a charcoal was obtained which possessed high absorptive power for such gases as chloropicrin and phosgene. Later this air process was changed to one in which steam is used; the cocoanut shell charcoal activated with steam was given the name “Dorsite.”
Complete apparatus for this air process was installed at the plant of the Astoria Light, Heat & Power Company, Long Island City, and the first charcoal was prepared during September, 1917. This was followed by a large amount of experimental work, relating to the raw material, the method of activation, and the type of furnace used. Because of the shortage of cocoanut hulls, it later became necessary to use a mixture of cocoanuts with cohune nuts, apricot and peach pits, cherry pits and vegetable ivory. Another substitute for cocoanut charcoal was found in a steam activated product from high grade anthracite coal, called “Batchite.”
The Offense Section and the Midland Section were concerned with the manufacture of mustard gas. This work was greatly delayed because of the unsatisfactory nature of the so-called chlorohydrin process. Another difficulty was the development of a satisfactory ethylene furnace. Finally in February, 1918, Pope in England discovered the sulfur chloride method of making mustard gas. At once all the energies of the Research Division were concentrated on this process, and in March steps were taken to put this process into production. An experimental plant was established at Cleveland; no attempt was made to manufacture mustard gas on a large scale, but the results obtained in the experimental studies were immediately transmitted to the manufacturing plants at Edgewood Arsenal, the Hastings-on-Hudson plant, the National Aniline & Chemical Company (Buffalo) plant, and the Dow Chemical Company (Midland) plant. The details of the work on mustard gas will be given in a later chapter. [Pg 63]
Special investigations were undertaken to develop a booster casing and adapter for 75 mm. gas shell, and to duplicate the French process of lining gas shell with glass.
The organization of the Development Division at the signing of the Armistice was as follows:
| Colonel F. M. Dorsey | Chief of the Division |
| Major L. J. Willien | Supt., Offense Section |
| Capt. O. L. Barnebey | Supt., Defense Section |
| Lt. Col. W. G. Wilcox | Supt., Experimental Station |
| Capt. Duncan MacRae | Special Investigation Section |
| Dr. A. W. Smith | Midland Section |
| Capt. J. R. Duff | Administrative Section |
The Proving Division had its origin in the decision to build an Experimental Ground for gas warfare under the direction of the Trench Warfare Section of the Ordnance Department. While this decision was reached about September, 1917, actual work on the final location (Lakehurst, N. J.) was not started until March 26, 1918, and the construction work was not completed until August 1, 1918. However, firing trials were started on April 25, 1918, and in all 82 were carried out.
The Proving Division was created to do two things: To experiment with gas shell before they reached the point where they could be manufactured safely in large numbers for shipment overseas; and to prove gas shell, presumably perfect and ready for shipment, to guard against any mechanical inaccuracies in manufacture or filling. It is evident that the second proposition is dependent upon the first. Shell can not be proved to ascertain the effect of gases under various conditions and concentrations until the mechanical details of the shell itself, purely an Ordnance matter, have been standardized. Unfortunately many of the tests carried out had to do with this very question of testing Ordnance.
For field concentration work two complete and separate lines of trenches were used and also several impact grounds. The trenches were built to simulate the trenches actually used in warfare. Each line of trench contained several concrete shell-proof dugouts and was also equipped with shelves into which boxes could be placed for holding the [Pg 64] sample bottles. At intervals of one yard throughout the trenches there were electrical connections available for electrical sampling purposes. The various impact grounds were used for cloud gas attacks, and experiments with mustard gas or in many cases for static trials. The samples were collected by means of an automatic sampling apparatus.
The work of the Division consisted in the first instance of determining the proper bursting charge. While a great deal of this work had been carried out in Europe, American gas shell were enough different to require that tests be carried out on them. The importance of this work is obvious, since phosgene, a substance with a low boiling point, would require a smaller bursting charge to open the shell and allow the substance to vaporize than would mustard gas, where the bursting charge must be not only sufficient to fragment the shell but also to scatter the liquid so that it would be atomized over the largest possible area. In the case of low boiling liquids it was necessary that the charge be worked out very carefully as a difference of one or two grams would seriously affect the concentration. Too small a charge would allow a cup to be formed by the base of the shell which would carry some of the liquid into the ground, while too great an amount of explosive tended to throw the gas too high into the air.
After the bursting charge had been determined a large number of shell were repeatedly fired into the trenches, wooded areas, rolling and level ground, and the concentration of gas produced and the effect upon animals placed within the area ascertained. From the results of these experiments the Proving Division was able to furnish the artillery with data regarding how many shell of given caliber should be used, with corrections for ranges, wind velocities, temperatures, ground conditions, etc. Trials were also held to determine how many high explosive (H.E.) shell could be fired with gas shell on the same area without unduly affecting the concentration. This was important, because H.E. shell were useful in disguising gas bombardments. Gas shell can usually be distinguished by the small detonation on bursting.
Experiments were performed to determine the decomposition of various [Pg 65] gases on detonation. The shell were fired at a large wooden screen and burst on impact. Samples of gas were taken immediately and analyzed.
Co-operative tests were carried out with the Gas Defense Division to determine the value of given masks under field conditions. Companies of infantry, fully equipped for the field, would wear masks for hours at a time digging trenches, cutting timber, drilling, etc., and imitating in every way, as far as possible, actual field conditions. During these activities tons of gas in cylinders were released in such a way that the men were enveloped in a far higher concentration than would probably ever be the case in actual battle. These tests gave valuable data for criticizing gas mask construction.
Another line of activity consisted of a study of the persistency and relative effectiveness of various samples of mustard gas, in which the liquid was distributed uniformly upon the surface of grassy zones one to three feet in width, which formed the periphery of circular areas 14 to 21 feet in diameter, the central part of each circle being occupied by animals.
The work of the Proving Division was brought to an end (by the Armistice) just at the time when it had reached its greatest usefulness. Not only were the physical properties and personnel of the Division developed to the maximum degree, but the production of gas shell in this country for shipment to France had just reached the stage where the Proving Ground could have been used to its fullest extent in their proving.
From the standpoint of the man at the front the Training Division is one of the most important. To him gas warfare is an ever present titanic struggle between poisonous vapors that kill on one side, and the gas mask and a knowledge of how and when to wear it, on the other. Because of this it is rather surprising that we did not hear more about this branch of the Service. It did exist, however, and credit must be given to those camp gas officers who remained in the United States performing an inconspicuous and arduous duty in the face of many local obstacles. [Pg 66]

Fig. 8.
The Field Training Division of the Gas Defense Service in the United States was organized in September, 1917, and consisted of Major J. H. Walton and 45 first lieutenants, all chemists. These men were given a three months’ military training at the American University. The arrival of Major (now Colonel) Auld during this time was very helpful, as he was able to give the Section first-hand knowledge. About 12 of the 45 men wore sent to France, while the remainder, together with British Gas Officers, were assigned to various Divisions still in training. There was little idea at that time as to what constituted real gas training. No one knew how much gas training would be received in France, and since little was often received due to lack of time, many men went into action with no idea of what this training really meant. Moreover, an [Pg 67] order that the gas officers should not go to France with their Divisions had, as was only natural, a discouraging effect upon the men and upon gas training and discipline generally.
In January, 1918, the gas officers were transferred to the Engineers, and designated as the 473d Engineers. Later an Army Gas School was established at Camp Humphreys. Because of the rapidly changing personnel, owing to overseas assignments, the policy was adopted of sending specialized gas officers only to Divisional Camps and the larger training centers. The need of a larger unit and increased authority was recognized by all intimately associated with the work, but little was accomplished until the transfer to the Chemical Warfare Service. Upon the appointment of Brigadier General H. C. Newcomer as Assistant Director of the Chemical Warfare Service, he was placed in charge of all military affairs of the Service, and the administrative officers of the Training Section became his “military assistants.” A few weeks later the Training Section of the Administration Division, C.W.S. was formed.
At this time new duties fell to the lot of this Section, among the more important being:
(1) The organization of gas troops and casual detachments for overseas duty;
(2) The establishment of a Chemical Warfare Training Camp;
(3) The procurement and training of officers for overseas duty.
For this purpose a training camp was established near the Proving Ground (Camp Kendrick) to hold 1300 officers and men. Line officers were sent from the larger camps for training, the best of whom might later be transferred to the Chemical Warfare Service for duty as Gas Officers.
The work of the Section eventually grew to such proportions that it was recognized as the Training Division of the Chemical Warfare Service. It differed from other Divisions in that all administrative routine was carried on through the offices of the Director, and with the assistance and co-operation of its various Sections.
Because of the formation of the Chemical Warfare Service and the [Pg 68] apparent need for officers, the office was soon flooded with applications for commissions. These were carefully examined and the men were sent first, by courtesy of the Chief of Engineers, to Camp Humphreys for a month’s course of military training. At the end of this period they were sent to Camp Kendrick as students of the Army Gas School. Toward the last of October all the officers and enlisted men were transferred to Camp Kendrick where an Officers’ Training Battalion was organized.
It is obvious that the gas training of troops was the most responsible duty of the Training Division. There was constantly in mind an ideal of supervised and standardized training for all troops in the United States, and the Division, at the time of the Armistice, for the first time found itself with a nearly adequate corps of officers through whom this ideal could be realized.
Dr. Yandell Henderson of Yale University was the logical man to inaugurate the medical work of the Bureau of Mines, because of his experience with oxygen rescue apparatus. A member of the first committee of the Bureau, he secured, in July, 1917, an appropriation for the study of toxic gases at Yale. This was in charge of Doctors Underhill, therapy; Marshall, pharmacology; and Winternitz, pathology. When the American University Station was opened Marshall was given charge of the pharmacology. About the same time a factory protection unit was organized under the direction of Doctors Bradley, Eyster and Loevenhart. At first this committee reported to the Ordnance Department, but later the work was transferred to the Gas Defense Service.
In December, 1917, the Medical Advisory Board was organized. This included all the men who were carrying on experimental work of a medical nature. This board had as its object the correlation of all medical work; new work was outlined and attempts were made to secure the co-operation of scientific men throughout the country. The following groups of workers assisted in this effort: At Yale, Underhill studied therapy, turning his animals over to Winternitz for pathological [Pg 69] study. Henderson was specially interested in the physiology of aviation. At the American University Marshall carried on pharmacological research, specially as regards mustard gas, the toxicology being covered by Loevenhart. A pathological laboratory was also started, under Winternitz, where many valuable studies were made.[14] At Cleveland Sollmann was busy with mustard gas and protective agents. Pearce, working in co-operation with Dr. Geer of the Goodrich Rubber Company, perfected the Goodrich Lakeside Mask. His study was very valuable as concerning the physiology of the gas mask. At Ann Arbor Warthin and Weller[15] were studying the physiology and pathology of mustard gas. Wells, Amberg, Helmholz and Austin of the Otho Sprague Memorial Institute were interested in protective clothing, while at Madison, Eyster, Loevenhart and Meek were engaged in a study of the chronic effect of long exposures to low concentrations, and later expanded their work to protective ointments and certain problems in pathology.
In the spring of 1918 many of these men were commissioned into the Gas Defense Service of the Sanitary Corps, and were later transferred to the Chemical Warfare Service as the Medical Division, with Colonel W. J. Lyster, M.C., in charge.
One of the most important functions of this Division was the daily testing of a large number of compounds for toxicity, lachrymatory or vesicant properties. The accuracy of these tests might and probably did save a large amount of unnecessary experimental work on the part of the Research Division. These tests are described in a later chapter.
Very interesting and likewise valuable was the study of mustard gas by Marshall, Lynch and Smith. They were able to work out the mechanism of its action and the varying degrees of susceptibility in individuals (see page 171).
Another interesting point was the fact that in the case of certain gases there is a cumulative effect. With superpalite and mustard gas the lethal concentration (that concentration which is fatal after a [Pg 70] given exposure) is lower on longer exposures. On the other hand there is no cumulative effect with hydrocyanic acid. Whether the action is cumulative or not depends on the rate at which the system destroys or eliminates the poison.
This chapter should not be closed without reference to the Liaison Service that was established between the United States and her Allies, especially England.
During the early days no one in the States was familiar with the details of gas warfare. At the request of the Medical Corps, upon the urgent representations of the Gas Service, A.E.F., Captain (now Major) H. W. Dudley was sent to this country (Sept., 1917) to assist in the development and manufacture of gas masks. For some time he was the Court of Appeal on nearly all technical points regarding matters of defense. Dudley’s continual insistence on the need for maintaining the highest possible standard of factory inspection was one of the factors resulting in the excellent construction of the American Mask. In March, 1918, Lieut. Col. Dewey and Captain Dudley made a trip to England and France, during which the idea of a liaison between the defense organizations of the two countries originated. Dudley was transferred to the Engineers, promoted and placed in charge of the Liaison service. While the time until the Armistice was too short to really test the idea, enough was accomplished to show the extreme desirability of some such arrangement.
Probably the best known liaison officer from the British was Colonel S. J. M. Auld, also sent upon the urgent representations of the Gas Service, A.E.F. He arrived in this country about the middle of October, 1917, in charge of 28 officers and 28 non-commissioned officers, who were to act as advisers in training and many other military subjects besides gas warfare. Since Auld had had personal experience with gas warfare as then practiced at the front, his advice was welcomed most heartily by all the different branches of the Army then handling gas warfare. On questions of general policy Auld was practically the sole [Pg 71] foreign adviser. The matter of gas training was transferred from the Medical Corps to the Engineers, and was greatly assisted by four pamphlets on Gas Warfare issued by the War College, which were prepared by Major Auld with the assistance of Captain Walton and Lieut. Bohnson. Later Auld gave the American public a very clear idea of gas warfare in his series of articles appearing in the Saturday Evening Post, and re-written as “Gas and Flame.”
Major H. R. LeSueur, who was at Porton previous to his arrival in this country in December, 1917, rendered valuable aid in establishing the Experimental Proving Ground and in its later operations.
Towards the close of the war the British War Office had drawn up a scheme for a Gas Mission, which was to correlate all the gas activities of England and America. This was never carried through because of the signing of the Armistice.
The French representatives, M. Grignard, Capt. Hanker and Lt. Engel furnished valuable information as to French methods, but they were handicapped by the fact that French manufacturers did not disclose their trade secrets even to their own Government.
About August, 1918, Lieut. Col. James F. Norris opened an office in London. His duties were to establish cordial and intimate relations not only with the various agencies of the British Government which were connected with gas warfare, but also with the various laboratories where experiments were being conducted, that important changes might be transmitted to America with the least possible delay. The English made Colonel Norris a member of the British Chemical Warfare Committee. Here again the signing of the Armistice prevented a full realization of the importance of this work.
[Pg 72]
It is worth noting here that the Chemical Warfare Service was organized as a separate service in the American Expeditionary Forces nearly ten months before it was organized in the United States, and that the organization in the United States as heretofore described was patterned closely on that found so successful in France.
Very soon after the United States declared war against the Central Powers, a commission was sent abroad to study the various phases of warfare as carried on by the Allies, and as far as possible by the enemy. Certain members of this commission gave attention to chemical warfare. One of those who did this was Professor Hulett of Princeton University. He, with certain General Staff officers, gathered what information they could in England and France concerning the gases used and methods of manufacturing them, and to a very slight extent the methods of projecting those gases upon the enemy. Some attention was paid to gas masks, but there being nobody on the General Staff, or anywhere else in the Regular Army, whose duty it was to look out particularly for chemical warfare materials, these studies produced no results.
As has already been stated, the Medical Department started the manufacture of masks, and the Bureau of Mines, under the leadership of the Director, Mr. Manning, began studies upon poisonous gases and the methods of manufacturing them just before or shortly after war was declared.
Nevertheless, although American troops left for France in May, 1917, it was not until the end of August—the 17th to be exact—that definite action was taken toward establishing a Chemical Warfare Service, or, as [Pg 73] it was then known, a Gas Service in the American Expeditionary Forces. On that date a cablegram was sent to the United States to the effect that it was desired to make Lieut. Col. Amos A. Fries, Corps of Engineers, Chief of the Gas Service, and requesting that no assignments to the regiment of gas troops authorized in the United States be made which would conflict with this appointment. On August 22d, Lieut. Col. Fries entered upon his duties as Chief of the Gas Service.
There were then in France about 30 miles from the German lines, some 12,000 American troops without any gas masks or training whatever in Chemical Warfare. Immediate steps were taken to teach the wearing of the masks, and English and French gas masks were obtained for them at the earliest possible moment. At the same time efforts were made to obtain officer personnel for the C. W. S., and to have sent to France a laboratory for making such emergency researches, experiments, and testing as might become necessary. From that time to the end of the war the C. W. S. continued to develop on broad lines covering research, development, and manufacture; the filling of shell and other containers with poisonous gases, smoke and incendiary materials; the purchase of gas masks and other protective devices, as well as the handling and supply of these materials in the field; the training of the Army in chemical warfare methods, both in offense and defense; and the organization, equipment and operation of special gas troops.
This gave an ideal organization whereby research was linked with the closest possible ties to the firing line, and where the necessities of the firing line were brought home to the supply and manufacturing branches and to the development and research elements of the Service instantly and with a force that could not have been obtained in any other manner. The success of the C. W. S. in the field and at home was due to this complete organization. To the Commander-in-Chief, General Pershing, is due the credit for authorizing this organization and for backing it up whenever occasion demanded. Other details of this work will be considered under the following heads: Administrative; Training; Chemical Warfare Troops; Supply; Technical; Intelligence; and Medical. [Pg 74]
The duties of administration covered those necessary for a general control of research, of supply, of training, and the operation of special gas troops. At first the Chief of the Gas Service comprised the whole of the Service since he was without personnel, material, rules, regulations, or anything else of a chemical warfare nature.
The experience in getting together this organization should be sufficient to insure that the United States will never place on any other man’s shoulders the burden of organizing a new and powerful service in the midst of war, 4,000 miles from home, without precedent, material, or anything else on which to base action. It is true the Americans had available the experience of the English and the French, and it should be said to the credit of both of these nations that they gave of their experience, their time, and their material with the greatest freedom and willingness, but just as Americans are Americans and were Americans in 1917, just so the methods of the French and English or of the enemy were not entirely suitable to American conditions.
If there is any one thing needed in the training of U. S. Army leaders of today and for the future, it is vision—vision that can foresee the size of a conflict and make preparations accordingly. We do not mean vision that will order, as happened in some cases, ten times as much material as could possibly be used by even 5,000,000 troops, but the sort of vision that could foresee in the fall of 1917 that 2,000,000 men might be needed in France and then make preparations to get materials there for those troops by the time they arrived.
In order to cover the early formative period of the C. W. S. in France and to show some of the difficulties encountered, the following running account is given of some of the early happenings without regard to the subdivisions under which they might properly be considered.
Assignment of Chief of the Gas Service. Sailing from the United States on the 23d of July, 1917, Fries arrived in Paris on the morning [Pg 75] of August 14, 1917, and was immediately assigned the task of organizing a highway service for the American Expeditionary Forces. Five days later and before the highway order was issued, he was asked what he would think if his orders were changed so as to make him Chief of the newly proposed Gas Service. Being given one night to think it over he told the General Staff he would undertake the work. The road work was immediately closed up and on the 22d of August the organization of a Gas Service was actively started.
At that time some information concerning gases and gas troops had been gathered by Colonel Barber of the General Staff. Likewise, Colonel (later Brigadier General) Hugh A. Drum had made a rough draft of an order accompanied by a diagram for the establishment of the Gas Service. This information was turned over to Fries who was told to complete the draft of the order, together with an organization chart, for the action of the Commander-in-Chief. After one and a half days had been put on this work the draft and chart were considered in good enough shape to submit to General Pershing, Commander-in-Chief.
First Trip to British Gas Headquarters. Noting that the proposed organization provided for the handling of 4-inch Stokes’ mortars by gas troops, General Pershing asked why this work could not be done by regular trench mortar companies. He was told that gas operations were too technical and dangerous to be intrusted to any but especially trained troops, and that, furthermore, it was understood that 4-inch Stokes’ mortars were used only by the British troops. General Pershing said, “You had better beat it to the British Gas Headquarters in the field and settle definitely that and certain other minor points.” Fries told him he was only too glad to do this, and, having completed preparations, left on the morning of August 25th with Colonel Church and Captain Boothby, both of the Medical Department, for St. Omer, Headquarters of the British Gas Service in the Field.
Colonel Church of the Medical Department had been in France nearly one and a half years prior to the entry of the United States into the war, and had taken sufficient interest in Gas Warfare to collect [Pg 76] considerable information and a number of documents from French sources bearing on the defensive side of the subject. Captain Boothby had done the same with the British, including a course in a British Gas Defense School. On this trip they took up the defensive side with the British, while Fries took up the offensive side of the Service. The latter included gases used, gas troops, and ammunition and guns used in Gas Warfare by the Artillery and other branches of the Service. The trip included a brief visit to the headquarters of the First British Army in the vicinity of Lens, where the British Gas Service had a large depot of offensive gas material.
Order Forming Service. Returning on the 28th of August the order, together with a chart organizing the Service, was completed and submitted to the General Staff. This was published as G. O. 31, September 3, 1917. As a result of a study of the information submitted by Colonel Barber and General Drum, together with his own observations of British organization and work, Fries decided it was advisable to make the Service cover as complete a scope as possible and to make the order very general, leaving details to be worked out as time and experience permitted. This proved to be a very wise decision, because the entire absence of gas knowledge among Americans either in France or the United States made it necessary to build from the bottom up and do it rapidly. At that time, and at all times since, it was found utterly impossible to separate the defensive side from the offensive side. Indeed, many of the worst troubles of the British with their Gas Service throughout nearly the whole war arose from such a division of duties in their Service. Thus, the development of masks must be kept parallel with the development of gases and methods of discharging them. Otherwise a new gas invented may penetrate existing masks and preparations be carried far towards using it before the development of masks are undertaken to care for the new gas. Obviously a gas which our own masks will not take care of cannot be safely used by our own troops until new masks are developed to protect against it.
American and British Masks. Just prior to Fries’s assignment as Chief of the Gas Service twenty thousand American-made masks or box [Pg 77] respirators were received from the United States. Through the energy of Captain Boothby several of these had been sent at once to the British for test. The test showed that the granules in the canisters were entirely too soft, the charcoal of poor quality, and more than all else, the fabric of the face piece was so pervious to gases that chloropicrin became unbearable to the eyes in less than a minute under the standard test used by the British. A cable containing this information had been framed and sent to the United States just prior to Fries’s appointment as Chief of the Service.
August 23d, the day after Fries took charge, it was decided to adopt the British mask or box respirator as the principal mask and the French M-2 as an emergency, both to be carried by the soldier, the French M-2, however, to be used only when the British mask became lost or unfit for use. A requisition for one hundred thousand of each was at once submitted and very shortly approved by the General Staff.
Getting Gas Supplies. It should be stated here that inasmuch as no Gas Service had been organized in the United States, no money appropriation had been made for it, thereby making it necessary for the Gas Service to obtain all its supplies through other departments ordinarily handling the same or similar materials. Thus defensive supplies were obtained through the Medical Department and offensive supplies through the Ordnance Department, while other miscellaneous equipment was obtained through the Engineer Department, the Quartermaster Department, or the Signal Corps. This procedure proved exceedingly embarrassing, cumbersome and inefficient. To begin with it was necessary to get some agreement between the departments as to what each would supply. This was very difficult, resulting in delays and consumption of time which was urgently needed on other work.
Not only was there trouble in getting orders accepted and started on the way but following them up became practically impossible. None of the Departments furnishing the materials were especially interested in them nor in many instances did they realize the vital nature of them. Accordingly in order to get any action it was necessary to continually follow up all orders and doing this through another department created [Pg 78] friction and misunderstanding. Officers of these departments took the attitude that the whole question of obtaining supplies should be left to them, once the requisition was turned in. This could not be done. The Chief of the Gas Service was absolutely responsible for gas supplies, and he fully realized that no excuses would be accepted, no matter who stood in the way. It was necessary to get action. Finally the matter was settled, some six months after the Service was organized, by giving the Chemical Warfare Service the right of direct purchase.
Purchase of Offensive Gas Supplies. Realizing the difficulty that would probably be encountered in getting supplies at all times from the British and French, two requisitions for offensive gas supplies to be purchased from the British were submitted on September 8th and 10th respectively. It would seem proper to state here that investigation showed the British gas organization to be far superior to the French. Indeed, the latter practically had no organization.
Consequently it was determined to purchase complete equipment for gas troops and for the defensive side of the service from the British and to make no attempt to produce new materials, methods or equipment until ample supplies of the standard equipment of the British were at hand or in process of manufacture or delivery. This was another exceedingly wise conclusion. No supplies of any kind were received from the United States for the next eight months, and then only masks and certain defensive supplies. Indeed, no cylinders, mortars, projectors or artillery shell containing gas were received from the United States until just before the Armistice, though gas had been available in the United States for months in large quantities, over 3,600 tons having been shipped in one ton containers to the English and French. The Ordnance material was what was lacking.
Obtaining Personnel. On September. 8, Colonel R. W. Crawford was assigned to duty with the Gas Service. This matter of obtaining personnel became immediately, and continued for almost a year to be, one of the most serious difficulties facing the new Gas Service. The troubles here again were the same as those in respect to supplies. None of the old departments were especially interested in gas and hence none of them desired to let good officers be transferred. [Pg 79]
Officers were scarce in the early days in France in every department of the Service, consequently a new department with no organization in the United States and no precedents or opportunities for promotion made the obtaining of officers almost a matter of impossibility. Further than this, while the Engineer Department was at first supposed to furnish most of the officer personnel, it failed to do so, apparently looking upon the Gas Service as an unimportant matter when compared with the regular work of the Engineers. It was necessary to make direct application to the Chief of Staff to obtain Colonel Crawford and shortly thereafter to cable directly to the United States for officers. A year later enough officers were obtained but only after the organization of a separate Service in the United States.
Supplies for Gas Troops. Colonel Crawford was at once put in Charge of all supplies for the Gas Service, including the location and construction of separate depots for that Service. Prior to this the General Staff had decided to have chemical supplies stored in depots separate from those of other supplies on account of the poisonous nature of the gases which might prove very annoying if leakage occurred near any other class of supplies. Colonel Crawford took hold of this work with zeal and energy and so conducted it as to relieve the Chief of the Gas Service of all anxiety in that matter. As before stated, on the 10th of September a requisition for a very large quantity of offensive supplies for gas troops was submitted to the General Staff for approval. Inasmuch as this involved approximately 50,000 gas cylinders, 50,000 Liven’s drums, with at least 20,000 Liven’s projectors and a large number of Stokes’ mortars and bombs, there was considerable difficulty in getting it approved. Finally Colonel Malone of the Training Section, who took an active interest in the Chemical Warfare Service, got it approved. Then began the difficulty of getting the order placed and of trying to expedite the filling of the order on time. These difficulties were never overcome until after the entire purchase of supplies was, as previously related, taken care of by the Gas Service.
First Inter-allied Gas Conference. The first inter-allied gas conference was held in Paris on September 16th, and consisted of [Pg 80] American, British, French, Italian, and Belgian delegates. The conference busied itself mainly with questions of the medical treatment of gassed cases and of defense against gas.
Mustard Gas. The principal topic under consideration at this conference was the effects of the new mustard gas first used at Ypres against the British on the nights of the 11th and 12th of July, 1917. The British suffered nearly 20,000 casualties from this gas during the first six weeks of its use, and were so worried over it that the start of the attacks carried out later in the fall of 1917 against Ypres were delayed several days. The casualties were particularly heavy because the smell of the gas was entirely new and not unpleasant and because of the delayed action of the gas, whereby men got no indication of its seriousness until 4 to 8 hours after exposure. For these reasons men simply took shelter from the bombardment without putting on masks or taking other precautions. As a result of the Paris conference a long cable was sent to the United States asking among other things that immediate report be made on the possibilities of producing ethylene chlorhydrin, one of the essentials in the manufacture of mustard gas by the only method then known.
Within two weeks after this conference, there occurred an incident which illustrates the very great danger in taking the views of any one man unless certain that he is in a position to be posted on all sides of the question under discussion. A high British official was asked what he had heard in regard to the new mustard gas, and what and how it was considered. He said with emphasis that the British had no further fear of it since they had learned what it was and how to take care of themselves and that it had ceased to be any longer a problem with them.
Fries, knowing what he did, was convinced that this did not represent the attitude of the British authorities who knew what the gas was doing, and the statement was not allowed to influence the American Gas Service in the least. This was a very fortunate thing as events later proved. It should also be added that a quite similar report was made by a French officer in regard to mustard gas some time in the month of [Pg 81] October. The French officer had more reason for his attitude than the British officer as up to that time mustard gas had not been largely used against the French. However, both cases simply emphasize the danger of accepting the views of any man who has seen but one angle of a problem so complicated as gas in war.
Training in Gas Defense. In the latter part of October seventeen young engineer officers, who had just arrived in France, were assigned to the Gas Service and were promptly sent to British Gas Schools for training in mask inspection, salvage and repair and in training men to wear masks and take other necessary precautions against gas in the field. It was also necessary at this time to establish gas training in the First Division, and Captain Boothby was assigned to that work.

Fig. 9.—Destroying Mustard Gas on the Battle Field.
It is important to note that the Gas Service had to begin operations immediately upon its organization although it had almost no facilities [Pg 82] of any kind to work with. At one and the same time it was necessary to decide upon the kinds of masks to be used and then to obtain them; to decide upon methods of training troops in gas defense and start at once to do it; to decide upon gases to be used and manufactured in the United States and then obtain and send the necessary data and finally to decide what weapons gas troops were to use and to purchase those weapons, since none of them existed in the United States. Worse still no one in the United States was taking any interest in them.
New Mask. About November 1, Major Karl Connell of the Medical Department, National Guard of New York, reported for duty in response to a cablegram that had been sent asking for him by name. It was intended to send him to a British School to learn the art of teaching gas defense. However, learning after a short talk with him that he had been interested in making masks for administering anæsthesia, there was at once turned over to him samples of all the masks in use by both the Allies and the Germans, with a view to getting his ideas for a new mask. Within two or three hours he suggested a new mask having a metal face piece with sponge rubber against the face and with a canister to be carried on the back of the head.
At that early date it was realized that a new mask must be invented which would be far more comfortable and give better vision than the British respirators adopted for use. Connell, thirty-six hours after reporting, had so far developed his idea that he was sent to Paris to make the first model, which he succeeded in doing in about three weeks. This first mask was good enough to risk testing in a high concentration of chlorine and while it leaked to some extent it indicated that the idea was sound. The problem then was to perfect the mask and determine how it could be produced commercially on the large scale necessary to equip an army.
Since the British at this time and practically throughout the war were much ahead of the French in all phases of gas warfare, Connell was sent to London. There he succeeded in getting additional models in such shape that one of them was sent to the United States during the first few days of January, 1918. Connell’s work and experiments were continued [Pg 83] so successfully that after a model had been submitted to the General Staff, as well as to General Pershing himself, one thousand were ordered to be made early in May with a view to an extensive field test preparatory to their adoption for general use in the United States Army.
In this connection, during November, 1917, a letter was written to the United States stating that while the Gas Service in France insisted on the manufacture of British respirators exactly as the British were making them, they desired to have experiments pushed on a more comfortable mask to meet the future needs of the Army.
The following four principles were set down in that letter: (a) That the mask must give protection and that experience had shown that suitable protection could only be obtained by drawing the air through a box filled with chemicals and charcoal. (b) That there must be clear vision and that experience to date indicated that the Tissot method of bringing the inspired air over the eyepieces was by far the best, (c) That the mask must be as comfortable as compatible with reasonable protection, and that this meant the mouthpiece and noseclip must be omitted. (d) That the mask must be as nearly fool proof as it could be made. That is, it should be of quick and accurate adjustment, in the dark or in the trenches, and be difficult to disarrange or injure once in position.
Gas Training and Battle of Picardy Plains. On March 21, 1918, as is known to everyone, the Germans began their great drive from Cambrai across the Picardy Plains to Amiens. While the battle was expected it came as a complete surprise so far as the tactics used, and the extent and force of the attack, were concerned. Lieutenant Colonel G. N. Lewis, who had been sent about March 1 to British Gas Schools, and had been assigned to one of the schools run by the Canadians, was thus just on the edge of the attack. This gave him an opportunity to actually observe some of that attack and to learn from eye-witnesses a great deal more. The school, of course, was abandoned hurriedly and the students ordered back to their stations. Lewis submitted two brief reports covering facts bearing on the use of gas and smoke by the Germans. These reports exhibited such a grasp of gas and smoke battle [Pg 84] tactics that he was immediately ordered to headquarters as assistant on the Defense side of gas work, that is, on training in gas defense. Up to that time no one had been able to organize the Defensive side of gas work in the way it was felt it must be organized if it were to prove a thorough success. A month later he was put at the head of the Gas Defense Section, and in two months he had put the Defense Division on a sound basis. He was then ordered to the United States to help organize Gas Defense Training there.

Fig. 10.—Close Burst
of a Gas Shell.
The 6th Marines in the Sommediene Sector
near
Verdun, April 30, 1918.
Cabled Report on Picardy Battle. Based partly on Colonel Lewis’s written and oral reports, and also on information contained in Intelligence dispatches and the newspapers, a cablegram of more than 300 words was drafted reciting the main features of the battle so far as they pertained to the use of gas. This cablegram ended with the statement that “the above illustrates the tremendous importance of comfort in a mask” and that “the future mask must omit the mouthpiece and noseclip.”
Keeping the General Staff Informed of Work. In the early part of May, 1918, the Americans arrived in the vicinity of Montdidier, south [Pg 85] of Amiens, on the most threatened point of the western front. It was on May 18, 1918, that the Americans attacked, took, and held against several counter-attacks the town of Cantigny. Shortly afterward they were very heavily shelled with mustard gas and suffered in one night nearly 900 casualties. Investigation showed that these casualties were due to a number of causes more or less usual, but also to the fact that the men had to wear the mask 12 to 15 hours if they were to escape being gassed. Such long wearing of the British mask with its mouthpiece and noseclip is practically an impossibility and scores became gassed simply through exhaustion and inability to wear the mask.
An inspector from General Headquarters in reporting on supplies and equipment in the First Division, stated that one of the most urgent needs was a more comfortable mask. The First Division suggested a mask on the principles of the new French mask which was then becoming known and which omitted the mouthpiece and noseclip. The efforts of the American Gas Service in France to perfect a mask without a mouthpiece and noseclip were so well known and so much appreciated that they did not even call upon the Gas Service for remark. The assistant to the Chief of Staff who drew up the memorandum to the Chief simply said the matter was being attended to by the Gas Service. This illustrates the value of keeping the General Staff thoroughly informed of what is being done to meet the needs of the troops on the firing line.
Then, as always, it was urged that a reasonably good mask was far more desirable than the delay necessary to get a more perfect one. Based on these experiences with mask development, the authors are convinced that the whole tendency of workers in general, in laboratories far from the front, is to over-estimate the value of perfect protection based on laboratory standards. It is difficult for laboratory workers to realize that battle conditions always require a compromise between perfection and getting something in time for the battle. It was early evident to the Gas Service in France that we were losing, and would continue to lose, vastly more men through removal of masks of the British type, due to discomfort and exhaustion, than we would from a more comfortable but [Pg 86] less perfect mask. In other words when protection becomes so much of a burden that the average man cannot or will not stand it, it is high time to find out what men will stand, and then supply it even at the expense of occasional casualties. Protection in battle is always relative. The only perfect protection is to stay at home on the farm. The man who cannot balance protection against legitimate risks has no business passing on arms, equipment or tactics to be used at the Front.
As early as September, 1917, gas training was begun in the First Division at Condrecourt. This training school became the First Corps School. Later a school was established at Langres known as the Army Gas School while two others known as the Second and Third Corps Gas Schools were established elsewhere. The first program of training for troops in France provided for a total period of three months. Of this, two days were allowed the Gas Service. Later this was reduced to six hours, notwithstanding a vigorous protest by the Gas Service. However, following the first gas attacks against the Americans with German projectors in March, 1918, followed a little later by extensive attacks with mustard gas, the A. E. F. Gas Defense School was established at the Experimental Field. Arrangements were made for the accommodation of 200 officers for a six-day course. The number instructed actually averaged about 150, due to the feeling among Division Commanders that they could not spare quite so many officers as were required to furnish 200 per week.
This school was conducted under the Commandant of Hanlon Field, Lieutenant Colonel Hildebrand, by Captain Bush of the British Service. This Gas Defense School became one of the most efficient schools in the A. E. F., and was developing methods of teaching that were highly successful in protecting troops in the field.
Failure of German Gas. The losses of the Americans from German gas attacks fluctuated through rather wide limits. There were times in the early days during training when this reached 65 per cent of the total casualties. There were other times in battle, when due to extremely severe losses from machine gun fire in attacks, that the proportion of gas losses to all other forms of casualties was very [Pg 87] small. On the whole the casualties from gas reached 27.3 of all casualties. This small percentage was due solely to the fact that when the Americans made their big attacks at San Mihiel and the Argonne, the German supply of gas had run very low. This was particularly true of the supply of mustard gas.

Fig. 11.—German Gas Alarms.
Fries was at the front visiting the Headquarters of the First Army and the Headquarters of the 1st, 3d, and 5th Corps from two days before the beginning of the battle of the Argonne to four days afterwards. He watched reports of the battle on the morning of the attack at the Army Headquarters and later at the 1st, 5th and 3d Corps headquarters in the order named. No reports of any gas casualties were received. This situation continued throughout the day. It was so remarkable that he told the Chief of Staff he could attribute the German failure to use gas to only one of two possible conditions; first, the enemy was out of gas; second, he was preparing some master stroke. The first proved to be the case as examination after the Armistice of German shell dumps [Pg 88] captured during the advance revealed less than 1 per cent of mustard gas shell. Even under these circumstances the Germans caused quite a large number of gas casualties during the later stages of the fighting in the Argonne-Meuse sector.
Evidently the Germans, immediately after the opening of the attack, or more probably some days before, began to gather together all available mustard gas and other gases along the entire western battle front, and ship them to the American sector. This conclusion seems justified because the enemy never had a better chance to use gas effectively than he did the first three or four days of the Argonne fight, and knowing this fact he certainly would never have failed to use the gas if it had been available. Had he possessed 50 per cent of his artillery shell in the shape of mustard gas, our losses in the Argonne-Meuse fight would have been at least 100,000 more than it was. Indeed, it is more than possible we would never have succeeded in taking Sedan and Mezieres in the fall of 1918.
Officers’ Training Camp. The first lot of about 100 officers were sent to France in July, 1918, with only a few days’ training, and in some cases with no training at all. Accordingly, arrangements were made to train these men in the duties of the soldier in the ranks, and then as officers. Their training in gas defense and offense followed a month of strenuous work along the above mentioned lines.
This camp was established near Hanlon (Experimental) Field, at a little town called Choignes. The work as laid out included squad and company training for the ordinary soldier, each officer taking turns in commanding the company at drill. They were given work in map reading as well as office and company administration.
This little command was a model of cleanliness and military discipline, and attracted most favorable comment from staff officers on duty at General Headquarters less than two miles distant. Just before the Armistice arrangements were made to transfer this work to Chignon, about 25 miles southeast of Tours, where ample buildings and grounds were available to carry out not alone training of officers but of soldiers along the various lines of work they would encounter, from the handling of a squad, to being Chief Gas Officer of a Division. [Pg 89]
Educating the Army in the Use of Gas. As has been remarked before, the Medical Department in starting the manufacture of gas masks and other defensive appliances, and the Bureau of Mines in starting researches into poisonous gases as well as defensive materials, were the only official bodies who early interested themselves in gas warfare. Due to this early work of the Bureau of Mines and the Medical Department in starting mask manufacture as well as training in the wearing of gas masks, the defensive side of gas warfare became known throughout the army very far in advance of the offensive side. On the other hand, since the Ordnance Department, which was at first charged with the manufacture of poisonous gases, made practically no move for months, the offensive use of gas did not become known among United States troops until after they landed in France.
Moreover, no gas shell was allowed to be fired by the artillery in practice even in France, so that all the training in gas the artillery could get until it went into the line was defensive, with lectures on the offensive.
The work of raising gas troops was not begun until the late fall of 1917 and as their work is highly technical and dangerous, they were not ready to begin active work on the American front until June, 1918.
By that time the army was getting pretty well drilled in gas defense and despite care in that respect were getting into a frame of mind almost hostile to the use of gas by our own troops. Among certain staff officers, as well as some commanders of fighting units, this hostility was outspoken and almost violent.
Much the hardest, most trying and most skillful work required of Chemical Warfare Service officers was to persuade such Staffs and Commanders that gas was useful and get them to permit of a demonstration on their front. Repeatedly Chemical Warfare Service officers on Division staffs were told by officers in the field that they had nothing to do with gas in offense, that they were simply defensive officers. And yet no one else knew anything about the use of gas. Gradually, however, by constantly keeping before the General Staff and others the results of gas attacks by the Germans, by the British, by [Pg 90] the French, and by ourselves, headway was made toward getting our Armies to use gas effectively in offense.
But so slow was this work that it was necessary to train men particularly how to appeal to officers and commanders on the subject. Indeed the following phrase, used first by Colonel Mayo-Smith, became a watchword throughout the Service in the latter part of the war—“Chemical Warfare Service officers have got to go out and sell gas to the Army.” In other words we had to adopt much the same means of making gas known that the manufacturer of a new article adopts to make a thing manufactured by him known to the public.

Fig. 12.—A Typical Shell Dump near the Front.
This work was exceedingly trying, requiring great skill, great patience and above all a most thorough knowledge of the subject. As illustrating some of these difficulties, the Assistant Chief of Staff, G-3 (Operations) of a certain American Corps refused to consider a recommendation to use gas on a certain point in the battle of the Argonne unless the gas officer would state in writing that if the gas was so used it could not possibly result in the casualty of a single American soldier. Such an attitude was perfectly absurd. [Pg 91]
The Infantry always expects some losses from our own high explosive when following a barrage, and though realizing the tremendous value of gas, this staff officer refused to use it without an absolute guarantee in writing that it could not possibly injure a single American soldier. Another argument often used was that a gas attack brought retaliatory fire on the front where the gas was used. Such objectors were narrow enough not to realize that the mere fact of heavy retaliation indicated the success of the gas on the enemy for everyone knows an enemy does not retaliate against a thing which does not worry him.
But on the other hand, when the value of gas troops had become fully known, the requests for them were so great that a single platoon had to be assigned to brigades, and sometimes even to whole Divisions. Thus it fell to the Lieutenants commanding these platoons to confer with Division Commanders and Staffs, to recommend how, when and where to use gas, and do so in a manner which would impress the Commanding General and the Staff sufficiently to allow them to undertake the job. That no case of failure has been reported is evidence of the splendid ability of these officers on duty with the gas troops. Efficiency in the big American battles was demanded to an extent unheard of in peace, and had any one of these officers made a considerable failure, it certainly would have been reported and Fries would have heard of it.
Equally hard, and in many cases even more so, was the work of the gas officers on Division, Corps and Army Staffs, who handled the training in Divisions, and who also were required to recommend the use of gas troops, the use of gas in artillery shell and in grenades, and the use of smoke by the infantry in attack. However, the success of the Chemical Warfare Service in the field with these Staff officers was just as great as with the Regiment.
To the everlasting credit of those Staff Officers and the Officers of the Gas Regiment from Colonel Atkisson down, both Staff Gas officers and officers of the Gas Regiment worked together in the fullest harmony with the single object of defeating the Germans. [Pg 92]
Chemical Warfare troops were divided into two distinct divisions—gas regiments and staff troops.

Fig. 13.—Firing a 155-Millimeter Howitzer.
The men are wearing gas masks to keep out the enemy gas fired at them in Oct., 1918.]
Staff Troops. The staff troops of the Chemical Warfare Service performed all work required of gas troops except that of actual fighting. They handled all Chemical Warfare Service supplies from the time they were unloaded from ships to the time they were issued to the fighting troops at the front, whether the fighting troops were Chemical Warfare or any other. They furnished men for clerical and other services with the Army, Corps and Division Gas Officers, and they manufactured poisonous gases, filled gas shells and did all repairing and altering of gas masks. Though these men received none of the glamour or glory that goes with the fighting men at the front, yet they performed services of the most vital kind and in many cases did work as dangerous and hair raising as going over the top in the face of bursting shell and screaming machine gun bullets. [Pg 93]
Think of the intense interest these men must have felt when carrying from the field of battle to the laboratory or experimental field, shell loaded with strange and unheard of compounds and which might any moment burst and end forever their existence! Or watch them drilling into a new shell knowing not what powerful poison or explosive it might contain or what might happen when the drill “went through”!
And again what determination it took to work 12 or 16 hours a day way back at the depots repairing or altering masks, and, as was done at Chateroux, alter and repair 15,000 masks a day and be so rushed that at times they had a bare day’s work of remodeled masks ahead. But they kept ahead and to the great glory of these men no American soldier ever had to go to the front without a mask. And what finer work than that of these men who, in the laboratory and testing room, toyed with death in testing unknown gases with American and foreign masks even to the extent of applying the gases to their own bodies.
Heroic, real American work, all of it and done in real American style as part of the day’s work without thought of glory and without hope of reward.
The First Gas Regiment. In the first study of army organization made by the General Staff it was decided to recommend raising under the Chief of Engineers one regiment of six companies of gas troops.
Shortly after the cable of August 17, 1917, was sent stating that Lieut. Colonel Fries would be made Chief of the Gas Service, the War Department promoted him to be Colonel of the 30th Engineers which later became the First Gas Regiment. At almost the same time, Captain Atkisson, Corps of Engineers, was appointed Lieut. Col. of the Regiment. Although Colonel Fries remained the nominal Commander of the regiment, he never acted in that capacity, for his duties as Chief of the Gas Service left him neither time nor opportunity. All the credit for raising, training, and equipping the First Gas Regiment belongs to Colonel E. J. Atkisson and the officers picked by him.
Immediately upon the formation of the Gas Service, the Chief urged that many more than six companies of gas troops should be provided. These [Pg 94] recommendations were repeated and urged for the next two months or until about the first of November, when it became apparent that an increase could not be obtained at that time and that any further urging would only cause irritation. The matter was therefore dropped until a more auspicious time should arrive. This arrived the next spring when the first German projector attack against United States troops produced severe casualties, exactly as had been forecasted by the Gas Service. About the middle of March, 1918, an increase from two battalions to six battalions (eighteen companies) was authorized. A further increase to three regiments of six battalions each (a total of fifty-four companies) was authorized early in September, 1918, after the very great value of gas troops had been demonstrated in the fight from the Marne to the Vesle in July.

Fig. 14.—Receiving and Transmitting Data
for Firing Gas Shell while Wearing Gas Masks.
Battlefield of the
Argonne, October, 1918.
No Equipment for Gas Troops. About the first of December a cablegram was received from the United States stating that due to lack of equipment the various regiments of special engineers recently authorized, including the 30th (Gas and Flame) would not be organized until the spring of 1918. An urgent cablegram was then sent calling [Pg 95] attention to the fact that gas troops were not service of supply troops but first line fighting troops, and consequently that they should be raised and trained in time to take the field with the first Americans going into the line. At this same time the 30th regiment was given early priority by the General Staff, A. E. F., on the priority lists for troop shipments from the United States. The raising of the first two companies was then continued under Colonel Atkisson at the American University in Washington.
About January 15 word was received that the Headquarters of the regiment and the Headquarters of the First Battalion together with Companies A and B of the 30th Engineers (later the First Gas Regiment) were expected to arrive very soon. Some months prior General Foulkes, Chief of the British Gas Service in the field, had stated that he would be glad to have the gas troops assigned to him for training. It was agreed that the training should include operations in the front line for a time to enable the American Gas Troops to carry on gas operations independently of anyone else and with entire safety to themselves and the rest of the Army.
Due to the fact that the British were occupying their gas school, the British General Headquarters were a little reluctant to take the American troops Feb. 1. However, General Foulkes made room for the American troops by moving his own troops out. He then placed his best officers in charge of their training and at all times did everything in his power to help the American Gas Troops learn the gas game and get sufficient supplies to operate with. Colonel Hartley, Assistant to General Foulkes, also did everything he could to help the American Gas Service. These two officers did more than any other foreign officers in France to enable the Chemical Warfare Service to make the success it did.
Second Battle of the Marne. The Chief of the Gas Service, following a visit to the British Gas Headquarters, and the Headquarters of the American 2d Corps then operating with the British, arrived on the evening of July 17, 1918, at 1st Corps Headquarters at La Ferte sous Jouarre about 10 miles southeast of Château-Thierry.
Two companies of the First Gas Regiment would have been ready in 48 [Pg 96] hours to put off a projector attack against an excellent target just west of Belleau Wood had not the 2d battle of the Marne opened when it did. It is said that General Foch had kept this special attack so secret that the First American Corps Commander knew it less than 48 hours prior to the hour set for its beginning. Certainly the Chief of the Gas Service knew nothing of it until about 9:00 p.m., the night of July 17th. Consequently the gas attack was not made. At that time so little was known of the usefulness of gas troops that they were started on road work. At Colonel Atkisson’s suggestion that gas troops could clean out machine gun nests, he was asked to visit the First Corps headquarters and take up his suggestion vigorously with the First Corps Staff.
Attacking Machine Gun Nests. Thereupon the Gas troops were allowed to try attacking machine gun nests with phosphorus and thermite. This work proved so satisfactory that not long afterwards the General Staff authorized an increase in gas troops from 18 companies to 54 companies, to be formed into three regiments of two battalions each. The 6 companies in France did excellent work with smoke and thermite during all the second battle of the Marne to the Vesle river, where by means of smoke screens they made possible the crossing of that river and the gaining of a foothold on the north or German side.
With the assembling of American troops in the sector near Verdun in September, 1918, the gas troops were all collected there with the exception of one or two companies and took a very active part in the capture of the St. Mihiel salient. It was at this battle that the Chemical Warfare Service really began to handle offensive gas operations in the way they should be handled. Plans were drawn for the use of gas and smoke by artillery and gas troops both. The use of high explosives in Liven’s bombs was also planned. Those plans were properly co-ordinated with all the other arms of the service in making the attack. Gas was to be used not alone by gas troops but by the artillery. Plans were made so that the different kinds of gases would be used where they would do the most good. While these plans and their execution were far from perfect, they marked a tremendous advance and [Pg 97] demonstrated to everyone the possibilities that lay in gas and smoke both with artillery and with gas troops.
Following the attack on the St. Mihiel salient, came the battle of the Argonne, where plans were drawn as before, using the added knowledge gained at St. Mihiel. The work was accordingly more satisfactory. However, the attempt to cover the entire American front of nine divisions with only six companies proved too great a task. Practically all gas troops were put in the front line the morning of the attack. Due to weather conditions they used mostly phosphorus and thermite with 4 inch Stokes’ mortars. Having learned how useful these were in taking machine gun nests, plans were made to have them keep right up with the Infantry. This they did in a remarkable manner considering the weight of the Stokes’ mortar and the base plates and also that each Stokes’ mortar bomb weighed about 25 pounds. There were cases where they carried these mortars and bombs for miles on their backs, while in other cases they used pack animals.

Fig. 15.—Setting Up a Smoke Barrage with Smoke Pots.
Not expecting the battle to be nearly continuous as it was for three weeks, the men, as before stated, were all put in the front line the [Pg 98] morning of the attack. This resulted in their nearly complete exhaustion the first week, since they fought or marched day and night during nearly the whole time. Taking a lesson from this, in later attacks only half the men were put in the line in the first place, no matter if certain sectors had to be omitted. Fully as good results were obtained because, as the men became worn out, fresh ones were sent in and the others given a chance to recuperate. Officers relate many different occurrences showing the discipline and character of these gas troops. On one occasion where a battalion of infantry was being held up by a machine gun nest, volunteers were called for. Only two men, both from the gas regiment, volunteered though they were joined a little later by two others from the same regiment, and these four took the guns. While it was not considered desirable for gas troops to attempt to take prisoners, yet the regiment took quite a number, due solely to the fact that they were not only with the advancing infantry but at times actually in front of it. On another occasion a gas officer, seeing a machine gun battalion badly shot up and more or less rattled, took command and got them into action in fine shape.
At this stage the Second Army was formed to the southeast of Verdun and plans were drawn for a big attack about November 14. The value of gas troops was appreciated so much that the Second Army asked to have British gas troops assigned to them since no American gas troops were available. Accordingly in response to a request made by the American General Headquarters, the British sent 10 companies of their gas troops. These reached the front just before the Armistice, and hence were unable to carry out any attacks there.
This short history of the operations of the First Gas Regiment covers only the high spots in its organization and work. It covers particularly its early troubles, as those are felt to be the ones most important to have in mind if ever it be necessary again to organize C. W. S. troops on an extensive scale. The Regiment engaged in nearly 200 separate actions with poisonous gases, smoke and high explosives, and took part in every big battle from the second battle of the Marne to the end of the War. They were the first American troops to train with [Pg 99] the British, and were undoubtedly the first American troops to take actual part in fighting the enemy as they aided the British individually and as entire units in putting off gas attacks, in February and March, 1918. It would be a long history itself to recite the actions in which the First Gas Regiment took part and in which it won distinction.[16]
No better summary of the work of this Regiment can be written than that of Colonel Atkisson in the four concluding paragraphs of his official report written just after the Armistice:
“The First Gas Regiment was made up largely of volunteers—volunteers for this special service. Little was known of its character when the first information was sent broadcast over the United States, bringing it to the attention of the men of our country. The keynote of this information was a desire for keen, red-blooded men who wanted to fight. They came into it in the spirit of a fighting unit, and were ready, not only to develop, but to make a new service. No effort was spared to make the organization as useful as the strength of the limited personnel allowed.
“The first unit to arrive in France moved to the forward area within eight weeks of its arrival, and, from that time, with the exception of four weeks, was continuously in forward areas carrying on operations. The third and last unit moved forward within six weeks of its arrival in France, and was continuously engaged until the signing of the Armistice.
“That the regiment entered the fight and carried the methods developed into execution where they would be of value, is witnessed by the fact that over thirty-five percent of the strength of the unit became casualties.
“It is only fitting to record the spirit and true devotion which prompted the officers and men who came from civil life into this Regiment, mastered the details of this new service, and, through their untiring efforts and utter disregard of self, made possible any success which the Regiment may have had. It was truly in keeping with the high ideals which have prompted our entire Army and Country in this conflict. They made the motto of ‘Service,’ a real, living, inspiring thing.”
[Pg 100]
As previously stated it was decided early that the Chemical Warfare Service should have a complete supply service including purchase, manufacture, storage and issue, and accordingly separate supply depots were picked out for the Gas Service early in the fall by Col. Crawford. Where practicable these were located in the same area as all other depots though in one instance the French forced the Gas Service to locate its gas shell and bomb depot some fifteen miles from the general depots through an unreasonable fear of the gas.
Manufacture of Gases. Due to the time required and the cost of manufacturing gases, an early decision became imperative as to what gases should be used by the Americans, and into what shells and bombs they should be filled. As there was no one else working on the subject the sole responsibility fell upon the Chief of the Gas Service. The work was further complicated by the fact that the British and French did not agree upon what gases should be used. The British condemned viciously Vincennite (hydrocyanic acid gas with some added ingredients) of the French, while the French stated that chloropicrin, used by the British principally as a lachrymator, was worthless. Fries felt the tremendous responsibility that rested upon him and finally after much thought and before coming to any conclusion, wrote the first draft of a short paper on gas warfare. In that paper he took up the tactical uses to which gases might be put and then studied the best and most available gases to meet those tactical needs.
Without stating further details it was decided to recommend the manufacture and use of chlorine, phosgene, chloropicrin, bromoacetone and mustard gas. As the gas service was also charged with handling smoke and incendiary materials, smoke was prescribed in the proportion of 5 per cent of the total chemicals to be furnished. The smoke material decided upon was white phosphorus.
The paper on Gas Warfare was then re-drafted and submitted to the French and British and written up in final form prescribing the gases [Pg 101] above mentioned on October 26. Following this a cable was drawn and submitted to the General Staff. After many conferences and some delay the cable went forward on November 3.
Cable 268, November 4, 1917
Paragraph 12. For chief of Ordnance. With reference to paragraph 2 my cablegram 181, desire prompt information as to whether recommendation is approved that phosgene, chloropicrin, hydrocyanic acid, and chlorine be purchased in France or England and filling plants established in France for filling shells and bombs with those gases.
Subparagraph A. Reference to your telegram 253, recommend filling approximately 10 per cent all shells with gases as given below, but that filling plants and gas factories be made capable of filling a total of 25 per cent. Unless ordinary name is given, gases are designated by numbers in chemical code War Gas investigations. Of 75 millimeter shells fill 1 per cent Vincennite, 4 per cent phosgene or trichloromethyl chloroformate, 2 per cent chloropicrin, 2½ per cent mustard gas, ½ per cent with bromoacetone and ½ per cent with smoke material. According to French 75 millimeter steel shells should not be filled with Vincennite more than three months before being used. No trouble with other gases or other sized shells except that bromoacetone must be in glass lined shells. Of 4.7 inch shells fill 5 per cent with phosgene or trichloromethyl chloroformate, 2 per cent with chloropicrin, 2½ per cent with mustard gas, ½ per cent with bromoacetone and ½ per cent with smoke material. Provide same percentage for all other shells up to and including 8 inch caliber as for 4.7 inch shells. 4 inch Stokes’ mortar will use same gases and smoke shells and in addition thermit. 8 inch projector bombs will use the same as the Stokes’ mortar and also oil to break into flame on bursting. Cloud gas cylinders will be filled with 50 or 60 per cent phosgene, mixed with 40 to 50 per cent chlorine, or phosgene and some other gas. Renew recommendation that filling plants be established in France to provide sudden shifts in gas warfare of all kinds, as well as for filling all 4 inch Stokes’ mortar bombs, 8 inch projector bombs and cloud gas cylinders. It is strongly recommended that efforts be made to produce white phosphorus on large scale for its usefulness both as smoke screens and to produce casualties.
Subparagraph B. For the Adjutant General of the Army. With reference to paragraph 2, my cablegram 181, desire information as to whether recommendation is approved that an engineer officer assisted by Professor Hulett be assigned to Gas Service in Washington to handle all orders and correspondence concerning gas. [Pg 102]
Subparagraph C. For Surgeon General. With reference to paragraph 2 your cablegram 205, and paragraph 2, my cablegram 181, what is status of chemical laboratory for France? Also have the 12 selected Reserve Officers for training in gas defense sailed for France?
Subparagraph D. With reference to paragraph 17 your cablegram 165 and paragraph 2 my cablegram 181, Tissot has constructed simpler model of his mask for attachment to any box. Have ordered 6 which will be completed in two weeks, 3 of which will be forwarded at once. A simple type such as this may prove useful for large number of troops. Letter of permission to manufacture Tissot masks being forwarded.
Subparagraph E. With reference to paragraph 8 your cablegram 143, and paragraph 4 your cablegram 247, in considering charcoal and other fillers for canister of box respirator it should be remembered that the front is very damp, the air being nearly saturated during greater part of winter, fall and spring.
This cable is given in full to show that not later than November 4, 1917, it was known in the United States not only what gases would be required but also in what shells, bombs, guns and mortars each would be used. While a small quantity of Vincennite was recommended in this cable, another cable sent within a month requested that no Vincennite whatever be manufactured. This decision as to gases and guns in which they were to be used, while very progressive, proved entirely sound and remained unchanged, with slight exceptions due to new discoveries, until the end of the war. Without a thorough understanding of tactics a proper choice of gases could not have been made. This fact emphasizes the necessity of having a trained technical army man at the head of any gas service.
Due to the absence of a Chemical Warfare Service in the United States at this time, a very great deal of the information sent from France, whether by cable or by letter, never reached those needing it.
Smoke. About the first of December after a study of results obtained by the British and the Germans in the use of smoke in artillery shells for screening purposes, the Gas Service decided that much more smoke than had been stated in cable 268 to the United States [Pg 103] was desirable. The General Staff, however, refused to authorize any increase, but did allow to be sent in a cable a statement to the effect that a large increase in smoke materials might be advisable for smoke screens, and that accordingly the amount of phosphorus needed in a year of war would probably be three or four times the one and a half million pounds of white phosphorus stated to have been contracted for by the Ordnance Department in the United States. This advanced position of the Gas Service in regard to smoke proved sound in 1918, when every effort was made to increase the quantity of white phosphorus available and to extend its use in artillery shells including even the 3 inch Stokes’ mortar.

Fig. 16.—Troops Advancing Behind a Smoke Barrage (Phosphorus).
Overseas Repair Section No. 1. During the latter part of November, 1917, Overseas Repair Section No. 1, under the command of Captain Mayo-Smith, Sanitary Corps, with four other officers and 130 men, arrived in France. Since mask development and manufacture in the United States was still under the Medical Department, this mask repair section was organized as a part of the Sanitary Corps. As there were at that time no masks to be repaired and no laboratory equipment or buildings for that purpose on hand and none likely to be for months to come, Captain Mayo-Smith was assigned to duty under Colonel Crawford, [Pg 104] Chief Gas Officer with the Line of Communication, in Paris. A site for a mask repair plant was located at Châteauroux, and a site for a gas depot at Gievres was investigated. Inasmuch as there was at that time greater need for men to learn the handling of poisonous gases than to repair masks, some 40 or 50 of the company were put in gas shell filling plants at Aubervilliers and Vincennes in the suburbs of Paris, while later still others were assigned to Pont de Claix near Grenoble. The remainder of the company were used in the Gas Depot at Gievres and in the office in Paris.
It was not until the latter part of June, 1918, that the mask repair plant began operations. In the meantime these men did very valuable work in shell filling and in learning the manufacture of gases. Several of them were sent to the United States, some of them remaining throughout the war to aid in gas manufacture and in shell filling.
Construction Division, Gas Service. The Construction Division under Colonel Crawford in Paris made complete plans for phosgene manufacturing plants, for shell filling plants and for the Mask Repair Plant. These plans included a complete layout of the work for all persons to be employed in the plants. During this same time a very careful study of the possibilities for manufacturing gas for filling shell in France was made.
Finally about March 1, in accordance with the strong recommendations of these men, Fries reported to General Pershing in person that the manufacture of gas as well as the filling of shell in France was inadvisable from every point of view and accordingly he recommended that gas manufacture and shell filling in France be given up. General Pershing strongly approved the recommendation and a cablegram was at once sent to the United States to that effect. The main reason for this action was the lack of chlorine, since chlorine was the principal ingredient of nearly all poisonous gases then in use. Chlorine takes, besides salt, electric power and lots of it. Electric power requires coal or water power. Neither of the latter sources were available in France. This question was gone into very thoroughly. The only place where power might have been developed was in a remote spot near Spain, and the outlook there was such that it appeared impossible to begin the manufacture of chlorine under two years. On the other hand the shipment [Pg 105] of chlorine from the United States required from 75 per cent to 100 per cent of the tonnage required to ship the manufactured gases themselves, to say nothing of the labor, raw materials, and the machinery that would have had to be shipped in order to manufacture gas in France.
Mustard Gas. As previously stated Mustard Gas was first used by the Germans against the British at Ypres on the nights of July 11 and 12, 1917. It was not used much against the French until more than two months later. Indeed, gas was never used by the Germans to the same extent against the French as against the English. There are probably two reasons for this; first, the Germans had a deeper hatred for the British than the French; second, the British morale was higher than the French in 1917, and the German thought that if he could break down this British morale, he could win the war.
The first attack came as a surprise and accordingly got an unusually large number of casualties. As previously stated the casualties numbered about 20,000 in about six weeks. This number was considered so serious that the beginning of the series of attacks against Ypres in the fall of 1917, was delayed by the British for 10 days or two weeks until they could study better how to avoid such great losses from mustard gas. While the composition of the gas was known within two or three days, as well as the laboratory method by which it was first manufactured by Victor Meyer in 1886, it took some 11 months to develop reliable and practical methods of manufacturing it on a large scale. The Inter-allied Gas Conference in September, 1917, gave a great deal of attention to mustard gas and methods of combating it both from the view point of prevention and of curing those gassed by it.
Just following the close of that conference a cable was sent to the United States asking the possibility of manufacturing ethylene chlorhydrin, the principal element in the manufacture of mustard gas by the only process then known. Later, that is about the middle of October, a cablegram was sent urging investigation into the manufacture of this gas. It is believed a great deal of time might have been saved had the policy of undue secrecy not been adopted by the British and others before the Americans entered the war. In fact we were only told [Pg 106] in whispers the formula for mustard gas, and where a description of it could be found in German chemistries. This was arrant nonsense since if the Germans had gotten all mustard gas information then in the hands of the British they would have received far less information than they already possessed on mustard gas.

Fig. 17.—“Who Said Gas?”
Whether the information sent to the United States on mustard gas ultimately proved of any great value is an open question since the methods adopted in the United States were very greatly superior to those used in England and in France. It probably helped by suggestion rather than by actual details of design. Anyhow it all emphasizes the difficulties encountered in war when so vital a substance as mustard gas must be investigated after the enemy has begun using it on a large scale.
Delay of British Masks. As December 1 approached, and as nothing further had been heard of the order for 300,000 British Respirators placed about the middle of October, a telegram was sent to England asking if deliveries would be made as required in the order for the [Pg 107] masks. This order required the first 75,000 to be delivered December 1, 1917. In reply it was stated that the British could not furnish these masks, and that they understood that the Americans were just beginning a large output of masks in the United States. An exchange of cablegrams with the United States showed that no masks could be expected from there for 3 to 5 months. Moreover it became increasingly evident that the Americans were going into the battle line sooner than at first contemplated. Another cablegram was then sent to England urging the delivery of these masks. The reply was to the effect that the English Government could not deliver the masks because they did not have enough for their own use. This situation was very serious. Unless the order for 300,000 masks placed with the British could be filled, we were facing the necessity of sending American troops into the front line with only the French M-2 mask. While the M-2 mask was then the only mask used by the French, it was well known to afford practically no protection against the high concentrations of phosgene obtained from cloud or projector attacks. And it was just such attacks as these that our men would encounter in the front line during training. Accordingly arrangements were made for a hurried trip to England.
Colonel Harrison of the British Royal Engineers was in charge of the British manufacture of masks and it is desired here to express appreciation of his uniform courtesy and great helpfulness. He exhibited their methods and facilities and assured us they could meet any requirements of ours for masks up to a half million, or even more if necessary, provided they were given time to establish additional facilities. Finally after a further exchange of cables the masks were obtained.
During December, 1917 and January, 1918, when every effort was being made to hurry a lot of masks from Havre—Havre being the British supply base in France from which the masks were issued to the United States, the severe cold and snow had so disorganized French traffic that it was extremely difficult to get cars moving at all. In an effort to get the masks, priority of shipment was obtained and two or three officers were assigned to convoy the cars. Notwithstanding convoying, one carload of [Pg 108] 4,000 masks, mainly threes and fours, became lost and only turned up five weeks later. To make matters worse the British were sending us very many more of the small sized No. 2 masks than we could use. The loss of this carload of 4,000 number threes and fours was all but a tragedy. Indeed, in order to get the First Brigade of the First Division equipped in time it was necessary to take a large number of masks already issued to men of the Second Brigade. These masks were first thoroughly washed and disinfected and then re-issued.
This all emphasizes the great difficulties that are encountered when a new and vital service must be organized in war 4,000 miles overseas without material, home supplies, or men to draw from. This struggle to get sufficient masks to keep all men fully equipped remained very acute until in July, 1918, when the arrival of hundreds of thousands of masks from the United States made the situation entirely safe. Even then the necessity of weakening the elastics and shortening the rubber tubing of the mouthpieces on some 700,000 masks, doubled up our work tremendously, and added enormously to our troubles in getting masks to the front in time.
Notwithstanding these troubles the Chemical Warfare Supply Service never failed and finally forged to the very forefront of all American supply services. Its method of issuing supplies to troops at the front has been adopted as the standard for American field armies of the future.
Gas Laboratory in Paris. Early in January, 1918, the first members of the Chemical Service Section, National Army, under the command of Colonel R. F. Bacon, arrived in France and reported for duty. Previously, a laboratory site at Puteaux, a suburb of Paris, had been selected. This plant had been built by a society for investigation into tuberculosis. Previous to the arrival of the Chemical Service Section, information had been requested from the United States by cable as to the size of the laboratory section to be sent over. The reply stated that the number would probably total about 100 commissioned and enlisted. The site at Puteaux was accordingly definitely decided upon. [Pg 109] Just following this decision two cables, one after the other, came from the United States recommending certain specified buildings in Paris for the laboratory. It was found upon investigation in both cases that the buildings were either absolutely unsuited or unfinished. This was another case of trying to fight a war over 4,000 miles of cable. Colonel Bacon was made head of the Technical Division, which position he held throughout the war.
Fig. 18.—Shaper for Opening Captured Gas Shell.
Technically Trained Men. In January, 1918, in response to a cable from the United States a request had been made on the French Government to send six of their ablest glass blowers to the United States to aid in making glass lined shells. The French Gas authorities said that it would be impossible to send those or indeed any other men trained in the manufacture or handling of poisonous gases or gas containers as they did not have enough such men for their own work. Accordingly a cablegram was drafted and sent to the United States, [Pg 110] requesting that 50 men experienced in various lines of technical and chemical work be sent to France. The French authorities said they would put them in any factories, laboratories or experimental places that the Chief of the Gas Service desired. A second inquiry about these men was sent but nevertheless no answer was ever received and no men were sent.
Protection Against Particulate Clouds. Just at this time, about the first of February, 1918, the danger that the Germans might devise some better method of sending over diphenylchloroarsine than by pulverizing it in high explosive shell was felt to be serious. The British had just then perfected protection against diphenylchloroarsine by employing unsized sulfate wood pulp paper—48 to 60 layers being required. This number of layers was found to be necessary as they are very thin and porous. The British had developed a method of putting this paper around a canister and yet keeping the canister small enough to fit into the knapsack by reversing its position therein; that is, putting the canister in the compartment of the knapsack made for the face piece and putting the face piece in the other compartment. Some of our own officers and enlisted men were sent to England to work with the British on this and an order given them for 200,000 of the protected canisters. They improved on the methods of the British and as it was found that sulfate paper was very scarce, investigations were made to see if any of it could be manufactured in France. Very soon thereafter such a place was located near the city of Nancy. Following this a cablegram was sent to the United States giving complete specifications for making this diphenylchloroarsine protection. From this cablegram successful samples were made though somewhat more bulky than those developed in England. Very few, however, of these were made in the United States due, we were informed, to the poor quality of the sulfate paper. Work was however begun energetically in the United States on other methods of protection against diphenylchloroarsine.
Numbers of Chemists Needed. It was figured that out of a total force of some 1,400 gas officers there would be needed in the A. E. F., exclusive of those in regiments, approximately 200 chemists, i.e., about 15 per cent of the whole. We arranged to have a good chemist on [Pg 111] each Division, Corps and Army Staff, and a certain number with the gas troops. It was proposed to put 20 to 40 in the laboratory in Paris and not to exceed 20 at the experimental field. This subject of personnel is touched on for the reason that a few people seem to have the idea that the Chemical Warfare Service should be made up of chemists exclusively. This is very far from being true. It was and is believed that the Chemical Warfare Service should be composed of men from every walk of life. In three positions out of every four in the field a good personality combined with energy, hard work and common sense count for more than mere technical training.
Hanlon (Experimental) Field. As early as December 15, 1917, it was decided that an experimental field in France was necessary, and a letter was written to the General Staff requesting authority to establish one. After considerable delay the authority was granted and search for a site begun. This was no easy task. While the French were loading millions of gas shells at the edge of Paris, they appeared unwilling at first to have us establish a gas experimental field except in abandoned or inaccessible spots. Finally a very good site was found and agreed to by the French some 7 miles south of General Headquarters. Just when we were ready to start work the French discovered that the proposed field included a portion of one of their artillery firing ranges. They then suggested another site within 3 miles of General Headquarters. This was a rather fortunate accident as the site suggested was a better one than at first picked out. The field was roughly rectangular from 7 to 8 miles in length, and 3 to 4 miles in width. The total area was about 20 square miles. The work of this experimental field proved a great success and was rapidly becoming the real center of the Gas Service in France.
The old saying that the history of a happy country is very brief applies to this story of the Technical Section of the Gas Service in France. Its work did not begin as early as that of the other sections, and as considerable of it was of a nature that could be put off without immediate fatal effects, the Section was enabled to grow without the very serious drawbacks encountered by other Sections of the Gas Service. [Pg 112]
Nevertheless its usefulness was very great. Those of the Technical Section either at the experimental field or at the laboratory were charged with the opening of all sorts of known and unknown gas and high explosive shells, fuses and similar things to determine their contents and their poisonous or explosive qualities. This was work of a very technical nature, and at the same time highly dangerous.
As stated elsewhere, the determination of the life of the masks became one of the problems which the laboratory was trying to solve. Hundreds of canisters were tested, and hundreds per month would have continued to have been tested throughout the remainder of the war had the war gone into 1919. It was on the Technical Section that devolved the duty of determining at the earliest possible moment the physical properties as well as the physiological effects of any new gas.
Also on that Section fell the preliminary reports as to the probable usefulness in war of a new gas whether sent over by the enemy or suggested by our own Technical men, or those of our Allies. This was indeed a task by itself, as it required a wide knowledge of the methods of using gases, methods of manufacturing them, and methods of projecting them on the field of battle.
In addition, it was the duty of the Technical Section to keep the Chief of the Service fully informed on all the latest developments in gases and to get that information in shape so that the Chief with his increasingly wide range of duties would be enabled to keep track of them without reading the enormous amount ordinarily written.
A much earlier start on technical work would have proved of immense advantage. In case of another war, the technical side of chemical warfare should be taken up with the very first expedition that proceeds to the hostile zone. Had that been done in France, we would have had masks and gases and proper shells and bombs at least six months before we did.
While Intelligence was for a long time under the Training or Technical Divisions, it finally assumed such importance that it was made a [Pg 113] separate Division. It was so thoroughly organized that by the time of the Armistice the Chief of the Division could go anywhere among the United States forces down to companies and immediately locate the Gas Intelligence officer.
Intelligence Division. This work was started by Lieutenant Colonel Goss within a month after he reported in October, 1917. The Intelligence Division developed the publication of numerous occasional pamphlets and also a weekly gas bulletin. So extensive was the work of this Division that three mimeograph machines were kept constantly going. The weekly bulletin received very flattering notice from the British Assistant Chief of Gas Service in the Field. He stated that it contained a great deal of information he was unable to get from any other source.
Among other work undertaken by this Intelligence Division was the compilation of a History of the Chemical Warfare Service in France. This alone involved a lot of work. In order that this history might be truly representative, about three months before the Armistice both moving and still pictures were taken of actual battle conditions, as well as of numerous works along the Service of Supplies.
Without going into further detail it is sufficient to say that when the Armistice was signed there were available some 200 still pictures, and some 8,000 feet of moving picture films. Steps were immediately taken to have this work continued along definite lines to give a complete and continuous history of the Chemical Warfare Service in France in all its phases.
The intelligence work of the Gas Service, while parallel to a small extent with the General Intelligence Service of the A. E. F., had to spread to a far greater extent in order to get the technical details of research, manufacture, development, proving, and handling poisonous gases in the field. It included also obtaining information at the seats of Government of the Allies, as well as from the enemy and other foreign sources.
The most conspicuous intelligence work done along these lines was by Lieutenant Colonel J. E. Zanetti, who was made Chemical Warfare liaison officer with the French in October, 1917. He gathered together and forwarded through the Headquarters of the Chemical Warfare Service to [Pg 114] the United States more information concerning foreign gases, and foreign methods of manufacturing and handling them, than was sent from all other sources combined. By his personality, energy and industry he obtained the complete confidence of the French and British. This confidence was of the utmost importance in enabling him to get information which could have been obtained in no other way. Suffice to say that in the 13 months he was liaison officer with the French during the war, he prepared over 750 reports, some of them very technical and of great length.
As a whole, the Intelligence Division was one of the most successful parts of the Chemical Warfare Service. Starting 2½ years after the British and French, the weekly bulletin and occasional papers sent out by the Chemical Warfare Service on chemical warfare matters came to be looked upon as the best available source for chemical warfare information, not alone by our own troops but also by the British.
The Medical Section of the Chemical Warfare Service was composed of officers of the Medical Department of the Army attached to the Chemical Warfare Service. These were in addition to others who worked as an integral part of the Chemical Warfare Service, either at the laboratory or on the experimental field in carrying out experiments on animals to determine the effectiveness of the gases.
The Medical Section was important for the reason that it formed the connecting link between the Chemical Warfare Service and the Medical Department. Through this Section, the Medical Department was enabled to know the kinds of gases that would probably be handled, both by our own troops and by the enemy, and their probable physiological effects.
Colonel H. L. Gilchrist, Medical Department, was the head of this Section. It was through his efforts that the Medical Department realized in time the size of the problem that it had to encounter in caring for gas patients. Indeed, records of the war showed that out of 224,089 men, exclusive of Marines, admitted to the hospitals in France, 70,552 were suffering from gas alone. These men received a total of [Pg 115] 266,112 wounds, of which 88,980, or 33.4 per cent, were gas. Thus ⅓ of all wounds received by men admitted to the hospital were gas. While the records show that the gas cases did not remain on the average in the hospitals quite as long as in the case of other classes of wounds, yet gas cases became one of the most important features of the Medical Department’s work in the field.
The Medical Section, through its intimate knowledge of what was going on in the Chemical Warfare Service as well as what was contemplated and being experimented with, was enabled to work out methods of handling all gas cases far in advance of what could have been done had there been no such section. One instance alone illustrates this fully. It became known fairly early that if a man who had been gassed with mustard gas could get a thorough cleansing and an entire change of clothing within an hour after exposure, the body burns could be eliminated or largely decreased in severity. This led to the development of degassing units. These consisted of 1,200 gallon tanks on five-ton trucks equipped with a heater. Accompanying this were sprinkling arrangements whereby a man could be given a shower bath, his nose, eyes and ears treated with bicarbonate of soda, and then be given an entire change of clothing. These proved a very great success, although they were not developed in time to be used extensively before the war closed.
There is an important side to the Medical Section during peace, that must be kept in mind. The final decision as to whether a gas should be manufactured on a large scale and used extensively on the field of battle depends upon its physiological and morale effect upon troops. In the case of the most powerful gases, the determination of the relative values of those gases so far as their effects on human beings is concerned is a very laborious and exacting job. Such gases have to be handled with extreme caution, necessitating many experiments over long periods of time in order to arrive at correct decisions.
[Pg 116]
Chlorine is of interest in chemical warfare, not only because it was the first poison gas used by the Germans, but also because of its extensive use in the preparation of other war gases. The fact that, when Germany decided upon her gas program, her chemists selected chlorine as the first substance to be used, was the direct result of an analysis of the requirements of a poison gas.
To be of value for this purpose, a chemical must satisfy at least the following conditions:
(1) It must be highly toxic.
(2) It must be readily manufactured in large quantities.
(3) It must be readily compressible to a liquid and yet be more or less easily volatilized when the pressure is released.
(4) It should have a considerably higher density than that of air.
(5) It should be stable against moisture and other chemicals.
Considering the properties of chlorine in the light of these requirements, we find:
(1a) Chlorine is fairly toxic, though its lethal concentration (2.5 milligrams per liter of air) is very high when compared with some of the later gases developed. This figure is the concentration necessary to kill a dog after an exposure of thirty minutes. Its effects during the first gas attack showed that, with no protection, the gas was very effective.
(2a) Chlorine is very readily manufactured by the electrolysis of a salt (sodium chloride) solution. The [Pg 117] operation is described below. In 100-pound cylinders, the commercial product sold before the War for 5 cents a pound. Therefore on a large scale, it can be manufactured at a very much smaller figure.
(3a) Chlorine is easily liquefied at the ordinary temperature by compression, a pressure of 16.5 atmospheres being required at 18° C. The liquid which is formed boils at -33.6° C. at ordinary atmospheric pressure, so that it readily vaporizes upon opening the valve of the containing cylinder. Such rapid evaporation inside would cause a considerable cooling of the cylinder, but this is overcome by running the outlet pipe to the bottom of the tank, so that evaporation takes place at the end of the outlet pipe.
(4a) Chlorine is 2.5 times as heavy as air, and therefore the gas is capable of traveling over a considerable distance before it dissipates into the atmosphere.
(5a) The only point in which chlorine does not seem to be an ideal gas, is in the fact that it is a reactive substance. This is best seen in the success of the primitive protection adopted by both the British and the French during the days immediately following the first gas attack.
At first, however, chlorine proved a very effective weapon. During the first six months of its use, its value was maintained by devising new methods of attack. When these were exhausted, phosgene was added (see next chapter). With the decline in importance of cloud gas attacks, and the development of more deadly gases, chlorine was all but discarded as a true war gas, but remained as a highly important ingredient in the manufacture of other toxic gases.
It was at first thought that the existing plants might be able to supply the government’s need of chlorine. The pre-war production averaged about 450 tons (900,000 pounds) per day. The greater amount of this was used in the preparation of bleach, only about 60,000 pounds per day being liquefied. Only a few of the plants were capable of even limited expansion. In an attempt to conserve the supply, the paper mills agreed to use only half as much bleach during the war, which arrangement added considerably to the supply available for war [Pg 118] purposes. It was soon recognized that even with these accessions, large additions would have to be made to the chlorine output of the country in order to meet the proposed toxic gas requirements.
After a careful consideration of all the factors, the most important of which was the question of electrical energy, it was decided to build a chlorine plant at Edgewood Arsenal, with a capacity of 100 tons (200,000 pounds) per day. The Nelson cell was selected for use in the proposed plant. During the process of erection of the plant, the Warner-Klipstein Chemical Company, which was operating the Nelson cell in its plant in Charleston, West Virginia, agreed that men might be sent to their plant to acquire the special knowledge required for operating such a plant. Thus when the plant was ready for operation, trained men were at once available.

Fig. 19.—Chlorine Plant, Edgewood Arsenal.
[Pg 119]
Fig. 20.—Ground Plan of Chlorine
Caustic Soda Plant,
Edgewood Arsenal.
[Pg 120] The following description of the plant is taken from an article by S. M. Green in Chemical and Metallurgical Engineering for July 1, 1919:
“The chlorine plant building, a ground plan of which is shown in Figure 20, consisted of a salt storage and treating building, two cell buildings, a rotary converter building, etc. In connection with the chlorine plant, there was also constructed a liquefying plant for chlorine and a sulfur chloride manufacturing and distilling plant.
“The salt storage and treating building was located on ground much below the cell buildings, which allowed the railroad to enter the brine building on the top of the salt storage tanks. These tanks were constructed of concrete. There were seven of these tanks, 34 feet long, 28 feet wide and 20 feet deep having a capacity for storing 4,000 tons of salt. There would have been 200 tons of salt used per day when the plant was running at full capacity.
“On the bottom of each tank distributing pipes for dissolving-water supply were installed, and at the top of each, at the end next to the building, there was an overflow trough and skimmer board arranged so that the dissolving-water after flowing up through the salt, overflowed into this trough and then into a piping system and into either of two collecting tanks. The system was so arranged that, if the brine was not fully saturated, it could be passed through another storage tank containing a deep body of salt. The saturated brine was pumped from the collecting tanks to any one of 24 treating tanks, each of which had a capacity of 72,000 gallons.
“The eighth storage bin was used for the storage of soda ash, used in treating the saturated brine. This was delivered from the bin on the floor level of the salt building to the soda ash dissolving tanks. From these tanks it was pumped to any one of the 24 treating tanks. After the brine was treated and settled, the clear saturated brine was drawn from the treating tanks through decanting pipes and delivered by pumps to any one of the four neutralizing tanks. These were located next to a platform on the level of the car body. This was to provide easy handling of the hydrochloric acid, which was purchased at first, though later prepared at the plant from chlorine and hydrogen. The neutralized brine was delivered from the tanks by a pump to a tank located at a height above the floor so that the brine would flow by gravity to the cells in the cell building.
“There were to be two cell buildings, each 541 feet long by 82 feet wide, and separated by partitions into four sections, containing six cell circuits of 74 cell units. Each section is a complete unit in itself, provided with separate gas pump, drying and cooling equipment, and has a guaranteed capacity of 12.5 tons of chlorine gas per 24 hours.
“Each Nelson electrolytic cell unit consists of a complete fabricated steel tank 13 by 32 by 80 inches, a perforated steel diaphragm spot welded to supporting angle irons, plate glass dome, fourteen Acheson [Pg 121] graphite electrodes 2.5 inches in diameter, 12 inches long and fourteen pieces of graphite 4 by 4 by 17 inches, and various accessories. (The cell is completely described in Chemical and Metallurgical Engineering, August 1st, 1919.) Each cell is operated by a current of 340 amperes and 3.8 volts and is guaranteed to produce 60 pounds of chlorine gas and 65 pounds of caustic soda using not more than 120 pounds of salt per 24 hours, the gas to be at least 95 per cent pure.
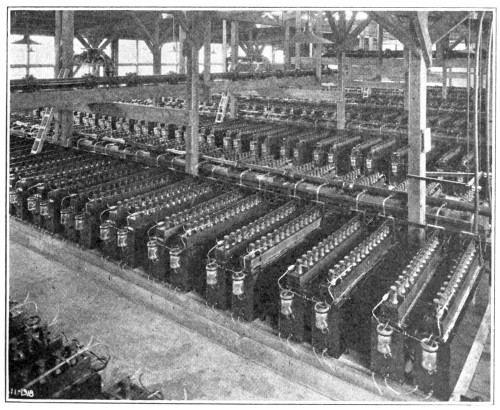
Fig. 21.—Interior View of the Cell Building.
“The salt solution from the cell feed tank, located in the salt treating building, flows by gravity through a piping system located in a trench running the length of each cell building, and is delivered to each cell unit through an automatic feeding device which maintains a constant liquor level in the cathode compartment.
“The remaining solution percolates from the cathode compartment through the asbestos diaphragm into the anode compartment and flows from the end of the cell, containing from 8 to 12 per cent caustic soda, admixed with 14 to 16 per cent salt, into an open trough and into a pipe in the trench and through this pipe by gravity to the weak caustic storage tanks located near the caustic evaporator building. [Pg 122]
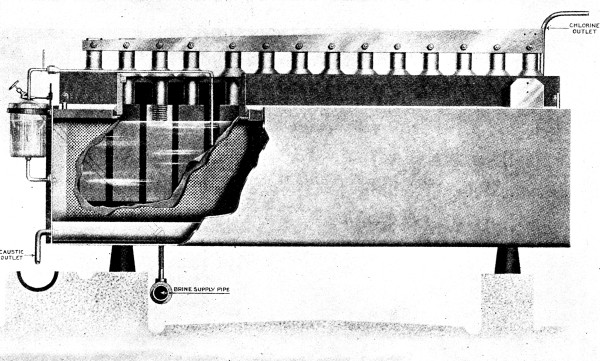
Fig. 22.—Nelson Electrolytic
Cell, showing the
Interior Arrangement of the Cell.
[Pg 123] “The gas piping from the individual cell units to and including the drying equipment is of chemical stoneware. The piping is so designed that the gas can be drawn from the cells through the drying equipment at as near atmospheric pressure as possible in order that the gas can be kept nearly free of air. When operating, the suction at the pump was kept at ¹/₂₀ inch or less. The quality of the gas was maintained at a purity of 98.5 to 99 per cent. The coolers used were very effective, the gas being cooled to within one degree of the temperature of the cooling water, no refrigeration being necessary. The drying apparatus consisted of a stoneware tower of special design containing a large number of plates, and thus giving a very large acid exposure. There was practically no loss of vacuum through the drying tower and cooler. The gas pumping equipment consisted of two hydroturbine pumps using sulfuric acid as the compressing medium. The acid was cooled by circulation through a double pipe cooler similar to those used in refrigerating work. The gas was delivered under about five pounds pressure into large receiving tanks located just outside the pump rooms, and from these tanks into steel pipe mains which conducted the gas to the chemical plant.”
The purity of the gas was such that it was not found necessary to liquefy it for the preparation of phosgene.
Chlorine, at ordinary atmospheric pressure and temperature, is a greenish yellow gas (giving rise to its name), which has a very irritating effect upon the membranes of the nose and throat. As mentioned above, at a pressure of 16.5 atmospheres at 18° C., chlorine is condensed to a liquid. If the gas is first cooled to 0°, the pressure required for condensation is decreased to 3.7 atmospheres. This yellow liquid has a boiling point of -33.6° C. at the ordinary pressure. If very strongly cooled, chlorine will form a pale yellow solid (at -102° C.). Chlorine is 2.5 times as heavy as air, one liter weighing 3.22 grams. 215 volumes of chlorine gas will dissolve in 100 volumes of water at 20°. It is very slightly soluble in hot water or in a concentrated solution of salt.
Chlorine is a very reactive substance and is found in combination in a [Pg 124] large number of compounds. Among the many reactions which have proved important from the standpoint of chemical warfare, the following may be mentioned:
Chlorine reacts with “hypo” (sodium thiosulfate) with the formation of sodium chloride. Hypo is able to transform a large amount of chlorine, so that it proved a very satisfactory impregnating agent for the early cloth masks.
Water reacts with chlorine under certain conditions to form hypochlorous acid, HOCl. In the presence of ethylene, this forms ethylene chlorhydrin, which was the basis for the first method of preparing mustard gas. In the later method, in which sulfur chloride was used, chlorine was used in the manufacture of the chloride.
Chlorine reacts with carbon monoxide, in the sunlight, or in the presence of a catalyst, to form phosgene, which is one of the most valuable of the toxic gases.
Chlorine and acetone react to form chloroacetone, one of the early lachrymators. The reaction of chlorine with toluene forms benzyl chloride, an intermediate in the preparation of bromobenzylcyanide.
In a similar way, it is found that the greater number of toxic gases use chlorine in one phase or another of their preparation. One author has estimated that 95 per cent of all the gases used may be made directly or indirectly by the use of chlorine.
Chlorine has been used in connection with ammonia and water vapor for the production of smoke clouds. The ammonium chloride cloud thus produced is one of the best for screening purposes. In combination with silicon or titanium as the tetrachloride it has also been used extensively for the same purpose.
On the other hand one may feel that, whatever bad reputation chlorine may have incurred as a poison gas, it has made up for it through the beneficial applications to which it has lent itself. Among these we may mention the sterilization of water and of wounds.
In war, where stationary conditions prevail only in a small number of cases, the use of liquid chlorine for sterilization of water is impractical. To meet this condition, an ampoule filled with chlorine [Pg 125] water of medium concentration has been developed, which furnishes a good portable form of chlorine as a sterilizing agent for relatively small quantities of water.
Chlorine has also been applied, in the form of hypochlorite, to the sterilization of infected wounds. The preparation of the solution and the technique of the operation were worked out by Dakin and Carrel. This innovation in war surgery has decreased enormously the percentage of deaths from infected wounds.
[Pg 126]
The first cloud attack, in which pure chlorine was used, was very effective, but only because the troops attacked with it were entirely unprotected. Later, in spite of the varied methods of attack, the results were less and less promising, due to the increased protection of the men and also to the gas discipline which was gradually being developed. During this time the Allies had started their gas attacks (Sept., 1915), and it soon became evident that, if Germany was to keep her supremacy in gas warfare, new gases or new tactics would have to be introduced.
The second poison gas was used in December, 1915, when about 20-25 per cent of phosgene was mixed with the chlorine. Here again the Germans made use of an industry already established. Phosgene is used commercially in the preparation of certain dyestuffs, especially methyl violet, and was manufactured before and during the war by the Bayer Company and the Badische Anilin und Soda Fabrik.
Phosgene can not be used alone in gas cylinders because of its high boiling point (8° C.). While this is considerably below ordinary temperatures, especially during the summer months, the rate of evaporation is so slow that a cloud attack could never be made with it alone. However, when a mixture of 25 per cent phosgene and 75 per cent chlorine, or 50 per cent phosgene and 50 per cent chlorine is used in warm weather there is no difficulty in carrying out gas attacks from cylinders. At the same time the percentage of phosgene in the mixture is sufficiently high to secure the advantages which it possesses. These advantages are at least three:
(a) Phosgene is more toxic than chlorine. It requires 2.5 [Pg 127] milligrams per liter of chlorine to kill a dog on an exposure of 30 minutes, but 0.3 milligram of phosgene will have the same effect. This of course means that a cloud of phosgene containing one-eighth (by weight) of the concentration of a chlorine cloud will have the same lethal properties.
(b) Phosgene is much less reactive than chlorine, so that the matter of protection becomes more difficult. Fortunately, word was received by the British of the intended first use of phosgene against them and consequently they were able to add hexamethylenetetramine to the impregnating solution used in the cloth masks.
(c) The third, and a very important, factor in the use of phosgene is the so-called delayed effect. In low concentrations, men may breathe phosgene for some time with apparently no ill effects. Ten or twelve hours later, or perhaps earlier if they attempt any work, the men become casualties.
Pure phosgene has been used in projector attacks (described in Chapter II). The substance has also been used in large quantities in shell; the Germans also used shell containing mixtures with superpalite (trichloromethyl chloroformate) or sneezing gas (diphenylchloroarsine).
Phosgene was first prepared by John Davy in 1812, by exposing a mixture of equal volumes of carbon monoxide and chlorine to sunlight; Davy coined the name “phosgene” from the part played by light in the reaction. While phosgene may be prepared in the laboratory by a number of other reactions, it was quite apparent that the first mentioned reaction is the most economical of these for large scale production. The reaction is a delicate one, however, and its application required extended investigation.
The United States was fortunate in that, for some months previous to the war, the Oldbury Electrochemical Company had been working on the utilization of their waste carbon monoxide in making phosgene. The results of these investigations were given to the government and aided considerably in the early work on phosgene at the Edgewood plant. [Pg 128]
Fig. 23.—Furnace for Generating Carbon Monoxide.
Of the raw materials necessary for the manufacture of phosgene, the chlorine was provided, at first by purchase from private plants, but later through the Edgewood chlorine plant. After a sufficient supply of chlorine was assured the next question was how to obtain an adequate supply of carbon monoxide. A method for this gas had not been developed on a large scale because it had never been necessary to make any considerable quantity of it. The French and English passed oxygen up through a gas producer filled with coke; the oxygen combines with the carbon, giving carbon monoxide. The oxygen was obtained from liquid air, for which a Claude liquid air machine may be used. The difficulty [Pg 129] with this method of preparing carbon monoxide was that the amount of heat generated was so great that the life of the generators was short. Our engineers conceived the idea of using a mixture of carbon dioxide and oxygen. The union of carbon dioxide with carbon to form carbon monoxide is a reaction in which heat is absorbed. Therefore by using the mixture of the two gases, the heat of the one reaction was absorbed by the second reaction. In this way a very definite temperature could be maintained, and the production of carbon monoxide was greatly increased.
Fig. 24.—Catalyzer Boxes Used in the Manufacture of Phosgene.
Carbon dioxide was prepared by the combustion of coke. The gas was washed and then passed into a solution of potassium carbonate. Upon heating, this evolved carbon dioxide.
Phosgene was then prepared by passing the mixture of carbon monoxide and chlorine into catalyzer boxes (8 feet long, 2 feet 9 inches deep and 11 inches wide), which are made of iron, lined with graphite and filled with a porous form of carbon. Two sets of these boxes were used. In the first the reaction proceeds at room temperature, and is about 80 [Pg 130] per cent complete. The second set of boxes were kept immersed in tanks filled with hot water, and there the reaction is completed.
The resulting phosgene was dried with sulfuric acid and then condensed by passing it through lead pipes surrounded by refrigerated brine.
The Germans prepared their phosgene by means of a prepared charcoal (wood or animal). Carbon monoxide was manufactured by passing carbon dioxide over wood charcoal contained in gas-fired muffles and was washed by passing through sodium hydroxide. This was mixed with chlorine and the mixture passed downward through a layer of about 20 cm. of prepared charcoal contained in a cast iron vessel 80 cm. in diameter and 80 cm. deep. By regulating the mixture so that there was a slight excess of carbon monoxide, the phosgene was obtained with only one-quarter of one per cent free chlorine. The charcoal (wood) was prepared by washing with hydrochloric and other acids until free from soluble ash; it was then washed with water and dried in vacuum. The size of the granules was about one-quarter inch mesh. Their life averaged about six months.
Phosgene is a colorless gas at room temperatures, but becomes a liquid at 8°. The odor of phosgene is suggestive of green corn or musty hay. One liter of phosgene vapor weighs 4.4 grams (chlorine weights 3.22 grams). At 0° C., the liquid is heavier than water, having a specific gravity of 1.432. At 25°, the vapor exerts a pressure of about 25 pounds per square inch. Phosgene is absorbed by solid materials, such as pumice stone and celite. Pumice stone absorbs more than its own weight of phosgene. Thus 5.7 grams of pumice absorbed 7.4 grams phosgene, which completely evaporated in 60 minutes. German shell have been found which contained such a mixture (phosgene and pumice stone). While the apparent reason for their use is to prevent the rapid evaporation of the phosgene, it is a question whether such is the case, for a greater surface is really present in the case of pumice stone than where the phosgene is simply on the ground. Phosgene is slowly [Pg 131] decomposed by cold water, rapidly by hot water. This reaction is important because there is always moisture in the air, which would tend to lower the concentration of the gas.
Phosgene is absorbed and decomposed by hexamethylenetetramine (urotropine). This reaction furnished the basis of the first protection used by the British. Later the catalytic decomposition of phosgene into carbon dioxide and hydrochloric acid by the charcoal in the mask furnished protection.
For most purposes a trace of chlorine in phosgene is not a disadvantage; for example, when it is used in cylinders or projectors. Under certain conditions, as when used as a solvent for sneezing gas, the presence of chlorine must be avoided, since it reacts with the substance in solution, usually producing a harmless material. Chlorine may be removed from phosgene by passing the mixture through cotton seed oil.
It was mentioned above that hexamethylenetetramine (urotropine) was used in the early pads (black veil and similar masks) and flannel helmets. This was found to be satisfactory against chlorine and phosgene, in the concentrations usually found during a cylinder attack. The mixture used consisted of urotropine, sodium thiosulfate (“hypo”), sodium carbonate and glycerine. The glycerine tended to keep the pads moist, while the other chemicals acted as protective agents against the mixture of phosgene and chlorine.
The introduction of the Standard Box Respirator with its charcoal-soda lime filling increased very materially the protection against phosgene. In this filling, the charcoal both absorbs the phosgene and catalyzes the reaction with the moisture of the air with which the phosgene is mixed, to form hydrochloric acid and carbon dioxide. Soda-lime absorbs phosgene but does not catalyze its decomposition. This shows the advantage of the mixture, since the hydrochloric acid, which is formed through the action of the charcoal, is absorbed by the soda-lime. Experiments seem to indicate that it does not matter which material is placed in the bottom of the canister, but that an intimate mixture is [Pg 132] the best arrangement. Using a concentration of 5,000 parts per million (20.2 mg. per liter) a type H canister (see page 217) will give complete protection for about 40 minutes; when the air-gas mixture passes at the rate of 16 liters per minute the efficiency or life of a canister increases with a decrease in temperature, as is seen in the following table (the concentration was 5,000 parts per million, the rate of flow 16 liters per minute)
| Temperature ° C. |
Efficiency (Time in minutes) |
|---|---|
| -10 | 223 |
| 0 | 172 |
| 10 | 146 |
| 20 | 130 |
| 30 | 125 |
| 40 | 99 |
From these figures it is seen that at -10° C. the life is about 50 per cent greater than at summer temperature. As would be expected the life of a canister is shortened by increasing the concentration of phosgene in the phosgene air mixture. This is illustrated by the following figures:
| Concentration p.p.m. |
Life (Time in minutes) |
|---|---|
| 5,000 | 177 |
| 10,000 | 112 |
| 15,000 | 72 |
| 20,000 | 58 |
| 25,000 | 25 |
(25,000 p.p.m. is equal to 101.1 mg. per liter.)
There is rather a definite relation between the concentration of the gas and the life of a canister at any given rate of flow. Many of these relations have been expressed by formulas of which the following is typical. At 32 liters per minute flow, C⁰˙⁹ × T = 101,840, in which C is the concentration and T the time.
The empty shell, after inspection, are loaded on trucks, together with the appropriate number of “boosters,” which screw into the top of the [Pg 133] shell and thereby close them. The trucks are run by an electric storage battery locomotive to the filling unit. The shell are transferred by hand to a conveyor, which carries the shell slowly through a cold room. During this passage of about 30 minutes, the shell are cooled to about 0° F. The cooled shell are transferred to shell trucks, each truck carrying 6 shell. These trucks are drawn through the filling tunnel by means of a chain haul operated by an air motor to the filling machine. Here the liquid phosgene is run into the shell by automatic machines, so arranged that the 6 shell are at the same time automatically filled to a constant void. The truck then carries the filled shell forward a few feet to a small window, at which point the boosters are inserted into the nose of the shell by hand. The final closing of the shell is then effected by motors operated by compressed air. The filling and closing machines are all operated by workmen on the outside of the filling tunnel.

Fig. 25.—Filling Livens’ Drums with Phosgene.
The filled shell are conveyed to the shell dump, where they are stored for 24 hours, nose down on skids, in order to test for leaks. [Pg 134]
Phosgene was first used in cloud attacks in December, 1915. These attacks continued for about nine months and were then gradually replaced, to a large extent, by gas shell attacks. Phosgene was first found in German projectiles in November, 1916. These shell were known as the D-shell. Besides pure phosgene, mixtures of phosgene and chloropicrin, phosgene and superpalite, and phosgene and diphenylchloroarsine have been found.

Fig. 26.—Interior of a Shell Dump.
The English introduced the use of projectors in the Spring of 1917. They have a decided advantage over shell in that they hold a larger volume of gas and readily lend themselves to surprise attacks. As the Germans say, “the projector combines the advantages of gas clouds and gas shell. The density is equal to that of gas clouds and the surprise effect of shell fire is also obtained.” [Pg 135]
Toward the close of the war, the Germans made use of a mixture of phosgene and pumice stone. A captured projector contained about 13 pounds of phosgene and 5½ pounds of pumice. There seems to be some question as to the value of such a procedure. Lower initial concentrations are secured; this is due, in part of course, to the smaller volume of phosgene in the shell containing pumice. Pumice does seem to keep the booster from scattering the phosgene so high into the air, and at the same time does not prevent the phosgene from being liberated in a gaseous condition. This would indicate that pumice gives a more even and uniform dispersion and a more economical use of the gas actually used.
Owing to its non-persistent nature (the odor disappears in from one and a half to two hours) and to its general properties, phosgene really forms an ideal gas to produce casualties.
Phosgene acts both as a direct poison and as a strong lung irritant, causing rapid filling of the lungs with liquid. The majority of deaths are ascribed to the filling up of the lungs and consequently to the suffocation of the patients through lack of air. This filling up of the lungs is greatly hastened by exercise. Accordingly, all rules for the treatment of patients gassed with phosgene require that they immediately lie down and remain in that position. They are not even allowed to walk to a dressing station. The necessity of absolute quiet for gassed patients undoubtedly partly accounts for the later habit of carrying out a prolonged bombardment after a heavy phosgene gas attack. The high explosive causes confusion, forcing the men to move about more or less and practically prevents the evacuation of the gassed. In the early days of phosgene the death rate was unduly high because of lack of knowledge of this action of the gas. Due to the decreased lung area for oxygenizing the air, a fearful burden is thrown on the heart, and accordingly, those with a heart at all weak are apt to expire suddenly when exercising after being gassed.
As an illustration of the delayed action of phosgene, a large scale raid made by one of the American divisions during its training is highly illuminating. [Pg 136]
This division decided to make a raid on enemy trenches which were situated on the opposite slope of a hill across a small valley. Up stream from both of the lines of trenches was a French village in the hands of the Germans. When the attack was launched the wind was blowing probably six or seven miles per hour directly down stream from the village, i.e., directly toward the trenches to be attacked. The usual high explosive box barrage was put around the trenches it was intended to capture.
Three hundred Americans made the attack. During the attack a little more than three tons of liquid phosgene was thrown into the village in 75- and 155-millimeter shells. The nearest edge of the village shelled with phosgene was less than 700 yards from the nearest attacking troops. None of the troops noticed the smell of phosgene, although the fumes from high explosive were so bad that a few of the men adjusted their respirators. The attack was made about 3 a.m., the men remaining about 45 minutes in the vicinity of the German trenches. The men then returned to their billets, some five or six kilometers back of the line. Soon after arriving there, that is in the neighborhood of 9 a.m., the men began to drop, and it was soon discovered that they were suffering from gas poisoning. Out of the 300 men making the attack 236 were gassed, four or five of whom died.
The Medical Department was exceedingly prompt and vigorous in the treatment of these cases, which probably accounted for the very low mortality.
This is one of the most interesting cases of the delayed action that may occur in gassing from phosgene. Here the concentration was slight and there is no doubt its effectiveness was largely due to the severe exercise taken by the men during and after the gassing.
It should be remarked in closing that while gas officers were not consulted in the planning of this attack, a general order was shortly thereafter issued requiring that gas officers be consulted whenever gas was to be used.
[Pg 137]
Without question the eyes are the most sensitive part of the body so far as chemical warfare is concerned. Lachrymators are substances which affect the eyes, causing involuntary weeping. These substances can produce an intolerable atmosphere in concentrations one thousand times as dilute as that required for the most effective lethal agent. The great military value of these gases has already been mentioned and will be discussed more fully later.
There are a number of compounds which have some value as lachrymators, though a few are very much better than all the others. Practically all of them have no lethal properties in the concentrations in which they are efficient lachrymators, though we must not lose sight of the fact that many of them have a high lethal value if the concentration is of the order of the usual poison gas. The lachrymators are used alone when it is desired to neutralize a given territory or simply to harrass the enemy. At other times they are used with lethal gases to force the immediate or to prolong the wearing of the mask.
A large number of the lachrymators contain bromine. In order to maintain the gas warfare requirements, it was early decided that the bromine supply would have to be considerably increased. The most favorable source of bromine is the subterranean basin found in the vicinity of Midland, Michigan. Because of the extensive experience of the Dow Chemical Co. in all matters pertaining to the production of bromine, they were given charge of the sinking of seventeen government wells, capable of producing 650,000 pounds of bromine per year. While the plant was not operated during the War, it was later operated to [Pg 138] complete a contract for 500,000 pounds of bromine salts. They will be held as a future war asset of the United States.
The principal lachrymators used during the War were:
Chloropicrin is something of a lachrymator, but it has greater value as a toxic gas.
One of the earliest lachrymators used was bromoacetone. Because of the difficulty of obtaining pure material, the commercial product, containing considerable dibromoacetone and probably higher halogenated bodies, was used. The presence of these higher bromine derivatives considerably decreased its efficiency as a lachrymator. The preparation of bromoacetone involved the loss of considerable bromine in the form of hydrobromic acid. This led the French to study various methods of preparation, and they finally obtained a product containing 80 per cent bromoacetone and 20 per cent chloroacetone, which they called “martonite.” As the war progressed, acetone became scarce, and the Germans substituted methylethylketone, for which there was little use in other war activities. This led to the French “homomartonite.”
Various other halogen derivatives of ketones have been studied in the laboratory, but none have proven of as great value as bromoacetone, either from the standpoint of toxicity or lachrymatory power.
Bromoacetone may be prepared by the action of bromine (liquid or vapor) upon acetone (with or without a solvent). Aqueous solutions of acetone, or potassium bromide solutions of bromine, have also been used.
Pure bromoacetone is a water clear liquid. There are great differences [Pg 139] in the properties ascribed to this body by different investigators. This probably is due to the fact that the monobromo derivative is mixed with those containing two or more atoms of bromine. A sample boiling at 126-127° and melting at -54°, had a specific gravity of 1.631 at 0°. It has a vapor pressure of 9 mm. of mercury at 20°.
While bromoacetone is a good lachrymator, it possesses the disadvantage that it is not very stable. Special shell linings are necessary, and even then the material may be decomposed before the shell is fired. The Germans used a lead-lined shell, while considerable work has been carried out in this country with enamel lined shell. Glass lined shell may also be used. It is interesting to note that, while bromoacetone decomposes upon standing in the shell, it is stable upon detonation. No decomposition products are found after the explosion, and even unchanged liquid is found in the shell. It may be considered as having a low persistency, since the odor entirely disappears from the surface of the ground in twenty-four hours.
Bromoacetone was also used by the Germans in glass hand grenades (Hand-a-Stink Kugel) and later in metal grenades. The metal grenades weighed about two pounds and contained about a pound and a half of the liquid.
Martonite was prepared by the French in an attempt more completely to utilize the bromine in the preparation of bromoacetone. They regenerated the bromine by the use of sodium chlorate:
NaClO₃ + 6HBr = NaCl + 3Br₂ + 3H₂O
In practice sulfuric acid is used with the sodium chlorate, so that the final products are sodium acid sulfate and a mixture of 20 per cent chloroacetone and 80 per cent bromoacetone, according to the reaction:
5(CH₃)₂CO + 4Br + H₂SO₄ + NaClO₃ =
4CH₂BrCOCH₃ + CH₂ClCO CH₃ + NaHSO₄ + 3H₂O.
This product is equally as effective as bromoacetone alone and is very much cheaper to manufacture. In general its properties resemble very closely those of bromoacetone. [Pg 140]
These two products were prepared by identical methods. About two-thirds of the product produced by the factory was prepared from methylethyl ketone which was obtained from the product resulting from the distillation of wood. The method employed was to treat an aqueous solution of potassium or sodium chlorate with acetone or methylethyl ketone, and then add slowly the required amount of bromine. The equation for the reaction in the case of acetone is as follows:
CH₃COCH₃ + Br₂ = CH₂BrCOCH₃ + HBr
Ten kg.-mols of acetone or methylethyl ketone were used in a single operation. About 10 per cent excess of chlorate over that required to oxidize the hydrobromic acid formed in the reaction was used. The relation between the water and the ketone was in the proportion of 2 parts by weight of the former to 1 part by weight of the latter. For 1 kg.-mol. wt. of the ketone, 10 per cent excess over 1 kg. atomic-weight of bromine was used.
The reaction was carried out either in earthenware vessels or in iron kettles lined with earthenware. The kettles were furnished with a stirrer made of wood, and varied in capacity from 4,000 to 5,000 liters. They were set in wooden tanks and cooled by circulating water. The chlorate was first dissolved in the water and then the ketone added. Into this mixture the bromine was allowed to run slowly while the solution was stirred and kept at a temperature of from 30° to 40° C. The time required for the addition of the bromine was about 48 hrs. When the reaction was complete, the oil was drawn off into an iron vessel and stirred with magnesium oxide in the presence of a small amount of water in order to neutralize the free acid. It was then separated and dried with calcium chloride. At this point a sample of the material was taken and tested. The product was distilled to tell how much of it boiled over below 130° when methylethyl ketone had been used. If less than 10 per cent distilled over, the bromination was considered to be satisfactory. If, however, a larger percentage of low boiling material was obtained, the product was submitted to further bromination. The material obtained in this way was found on analysis to contain slightly less than the theoretical amount of monobromoketone.
It was finally transferred by suction or by pressure into tank-wagons. At first lead-lined tanks were used, but later it was found that tanks [Pg 141] made of iron could be substituted. In order to take care of the small amount of hydrobromic acid, which is slowly formed, a small amount of magnesium oxide was added to the material. The amount of the oxide used was approximately in the proportion of 1 part to 1000 parts of ketone. When the magnesium oxide was used, it was found that the bromoketone kept without appreciable decomposition for about 2 months. The yield of the product from 580 kg. of acetone (10 kg.-mol. wts.) was 1,100 kg. The yield from 720 kg. of methylethyl ketone (10 kg.-mol. wts.) was 1,250 kg.
The use of ethyl iodoacetate was advocated at a time when the price of bromine seemed prohibitive. Because of the relative price of bromine and iodine under ordinary conditions, it is not likely that it would be commonly used. However, it is an efficient lachrymator and is more stable than the halogenated ketones, so that on a smaller scale it might be advisable to use it.
It is prepared by the reaction of sodium iodide upon an alcoholic solution of ethyl chloroacetate. It is a colorless oil, boiling at 178-180° C. (69° C. at 12 mm.) and having a density of about 1.8. It is very much less volatile than bromoacetone, having a vapor pressure of 0.54 mm. of mercury at 20° C. Ethyl iodoacetate is about one-third as toxic as bromoacetone, but has about the same lachrymatory value.
“Benzyl bromide” was also used during the early part of the war, usually mixed with bromoacetone. The material was not pure benzyl bromide, but the reaction product of bromine upon xylene, and should perhaps be referred to as “xylyl bromide.”
Pure benzyl bromide is a colorless liquid, boiling at 198-199° C., and having an odor reminiscent of water cress and then of mustard oil. The war gas is probably a mixture of mono- and dibromo derivatives, boiling at 210-220° C., and having a density at 20° C. of 1.3. The mixture of benzyl and xylyl bromides used by the Germans was known as “T-Stoff,” [Pg 142] while the mixture of 88 per cent xylyl bromide and 12 per cent bromoacetone was called “Green T-Stoff.”
As in the case of the halogenated acetones, it is necessary to use lead lined shell for these compounds. Enamel and glass lined shell may be used and give good results. While they are difficult of manufacture, satisfactory methods were being developed at the close of the war.
“T-Stoff” may be detected by the nose in concentrations of one part in one hundred million of air, and will cause profuse lachrymation with one part in a million. It is a highly persistent material and may last, under favorable circumstances, for several days. While it is relatively non-toxic, French troops were rendered unconscious by it during certain bombardments in the Argonne in the summer of 1915.
A number of derivatives of the benzyl halides have been tested and some have proven to be very good lachrymators. The difficulty of their preparation on a commercial scale has made it inadvisable to use them, and especially inasmuch as bromobenzyl cyanide has proven to be such a valuable compound.
Bromobenzyl cyanide is, chemically, α-bromo-α-tolunitrile, or phenyl-bromo-acetonitrile, C₆H₅CHBrCN. It is prepared by the action of bromine upon benzyl cyanide.
Benzyl cyanide is prepared by the action of sodium cyanide upon a mixture of equal parts of 95 per cent alcohol and benzyl chloride. The benzyl chloride in turn is obtained by the chlorination of toluene at 100°. The material must be fairly pure in order that the benzyl cyanide reaction may proceed smoothly. The cyanide is subjected to a fractional distillation and that part boiling within 3 degrees (the pure product boils at 231.7° C.) is treated with bromine vapor mixed with air. It has been found necessary to catalyze the reaction by sunlight, artificial light or the addition of a small amount of bromobenzyl cyanide.
The product obtained from this reaction, if the hydrobromic acid which [Pg 143] is formed is carefully removed by a stream of air, is sufficiently pure for use as a lachrymator. It melts from 16 to 22° C., while the pure product melts at 29° C. It cannot be distilled, even in a high vacuum. It has a low vapor pressure and thus is a highly persistent lachrymator.
Bromobenzyl cyanide is about as toxic as chlorine, but is many times as effective a lachrymator as any of the halogenated ketones or aromatic halides studied. It has a pleasant odor and produces a burning sensation on the mucous membrane.
Like the other halogen containing compounds, lead or enamel lined shell are necessary for preserving the material any length of time. In all of this work the United States was at a very marked disadvantage. While the Allies and the Germans could prepare substances of this nature and use them in shell within a month, the United States was sure that shell filled at Edgewood Arsenal probably would not be fired within three months. This means that much greater precautions were necessary, both as to the nature of the shell lining and as to the purity of the “war gas.”
The question of protection against lachrymatory gases was never a serious one. During the first part of the war this was amply supplied by goggles. Later, when the Standard Respirator was introduced, it was found that ample protection was afforded against all the lachrymators. Their principal value is against unprotected troops and in causing men to wear their masks for long periods of time.
The comparative value of the various lachrymators mentioned above is shown in the following table:
| Bromobenzyl cyanide | 0.0003 |
| Martonite | 0.0012 |
| Ethyl iodoacetate | 0.0014 |
| Bromoacetone | 0.0015 |
| Xylyl bromide | 0.0018 |
| Benzyl bromide | 0.0040 |
| Bromo ketone | 0.011 |
| Choroacetone | 0.018 |
| Chloropicrin | 0.019 |
The figures give the concentration (milligram per liter of air) necessary to produce lachrymation. The method used in obtaining these figures is given in Chapter XXI.
[Pg 144]
During the spring of 1917, strange reports came from the Italian front that the Germans were using a new war gas. This gas, while it did not seem to be very poisonous, had the combined property of being a lachrymator and also of causing vomiting. Large number of casualties resulted through the men being forced to remove their masks in an atmosphere filled with lethal gases. The gas had the additional and serious disadvantage of being a very difficult one to remove completely in the gas mask. The first American masks were very good when chlorine or phosgene was considered but were of no value when chloropicrin was used.
One of the interesting facts of chemical warfare is that few if any new substances were discovered and utilized during the three years of this form of fighting. Chlorine and phosgene were well known compounds. And likewise, chloropicrin was an old friend of the organic chemist. So much so, indeed, that several organic laboratories prepared the compound in their elementary courses.
Chloropicrin was first prepared by the English chemist, Stenhouse, in 1848, by the action of bleaching powder upon a solution of picric acid. This was followed by a careful study of its physical and chemical properties, few of which have any connection with its use as a poison gas. The use of picric acid as an explosive made it very desirable that other raw materials should be used. Chloroform, which is the ideal source theoretically (since chloropicrin is nitro-chloroform, Cl₃CNO₂), gave very poor yields. While it may be prepared from acetone, in fair yields, acetone was about as valuable during the war as was picric acid. Practically all the chloropicrin used was prepared from this acid as the raw material. [Pg 145]
In the manufacture of chloropicrin the laboratory method was adopted. This consisted simply in passing live steam through a mixture of picric acid and bleaching powder. The resulting chloropicrin passes out of the still with the steam. There was a question at first whether a steam jacketed reaction vessel should be used, and whether stirrers should be introduced. Both types were tested, of which the simpler form, without steam jacket or stirrer, proved the more efficient.
Fig. 27.—Interior of Chloropicrin Plant.
The early work was undertaken at the plant of the American Synthetic Color Company at Stamford, Connecticut. Later a large plant was constructed at Edgewood Arsenal. At the latter place ten stills, 8 by 18 feet, were erected, together with the necessary accessory equipment. The following method of operation was used:
The bleach is mixed with water and stirred until a cream is formed. This cream is then pumped into the still along with a solution of [Pg 146] calcium picrate (picric acid neutralized with lime). When the current of live steam is admitted at the bottom of the still, the temperature gradually rises, until at 85° C. the reaction begins. The chloropicrin passes over with the steam and is condensed. Upon standing, the chloropicrin settles out, and may be drawn off and is then ready for filling into the shell. The yield was about 1.6 times the weight of picric acid used.
Chloropicrin is a colorless oil, which is insoluble in water, and which can be removed from the reaction by distillation with steam. It boils at 112° C. and will solidify at -69° C. At room temperature it has a density of 1.69 and is thus higher than chloroform (1.5) or carbon tetrachloride (1.59). At room temperature it has a vapor pressure of 24 mm. of mercury. It thus lies, in persistency, between such gases as phosgene on the one hand, and mustard gas on the other, but so much closer to phosgene that it is placed in the phosgene group.
Chloropicrin is a very stable compound and is not decomposed by water, acids or dilute alkalies. The reaction with potassium or sodium sulfite, in which all the chlorine is found as potassium or sodium chloride, has been used as an analytical method for its quantitative determination. The qualitative test usually used consists in passing the gas-air mixture through a heated quartz tube, which liberates free chlorine. The chlorine may be detected by passing through a potassium iodide solution containing starch, or by the use of a heated copper wire gauze, when the characteristic green color is obtained.
An interesting physiological test has also been developed. The eye has been found to be very sensitive to chloropicrin. The gas affects the eye in such a way that its closing is practically involuntary. A measurable time elapses between the instant of exposure and the time when the eye closes. Below 1 or 2 parts per million, the average eye withstands the gas without being closed, though considerable blinking may be caused. Above 25 parts, the reaction is so rapid as to render proper timing out of the question. But with concentrations between 2 [Pg 147] and 25 parts, the subject will have an overpowering impulse to close his eye within 3 to 30 seconds. The time may be recorded by a stop watch and from the values thus determined a calibration curve may be plotted, using the concentration in parts per million and the time to zero eye reaction. Typical figures are given below. It will be noted that different individuals will vary in their sensitivity, though the order is the same.
| Conc. p.p.m. |
A Seconds |
B Seconds |
|---|---|---|
| 20.0 | 4.0 | 5.0 |
| 15.0 | 5.4 | 5.4 |
| 10.0 | 7.5 | 7.5 |
| 7.5 | 9.0 | 10.0 |
| 5.0 | 13.0 | 15.0 |
| 2.5 | 18.0 | 30.0 |
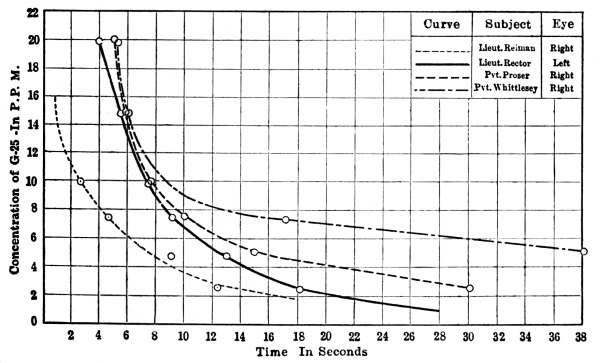
Fig. 28.—Calibration Curve of Eyes for Chloropicrin.
Because of the stability of chloropicrin, the question of protection resolves itself into finding an absorbent which is very efficient in [Pg 148] removing the gas from air mixtures. Fortunately such an agent was found in the activated charcoal used in the American gas mask. The removal of the gas appears to take place in two stages. In the first, the gas is adsorbed in such a way that the long-continued passage of air does not remove it. In the second, the gas is absorbed, and this, really excess gas, is removed by pure air passing over the charcoal. The relation of these two factors has an important bearing on the quality of charcoal to be used in gas masks. It appears that up to a certain point an increase of the quality is desirable: beyond this, it is of doubtful value.
Unlike phosgene, chloropicrin is absorbed equally well at all temperatures. Moisture on the other hand has a very decided effect. It appears that charcoal absorbs roughly equivalent weights of chloropicrin and of water; the presence of water in the charcoal thus displaces an approximately equal amount of chloropicrin.
In the study of canisters it has been found that the efficiency time is approximately inversely proportional to the concentration. Formulas have been calculated to express the relation existing between concentration and life of the canister, and also between the rate of flow of the gas and the life.
While water seems to have a decidedly marked effect upon the life of a canister, this is not true of other gases, and the efficiency of the canister for each gas is not decreased when used in a binary mixture.
Because of the high boiling point of chloropicrin it can only be used in shell. The German shell usually contained a mixture of superpalite (trichloromethyl chloroformate) and chloropicrin, the relative proportions being about 75 to 25. These were called Green Cross Shell, from the peculiar marking on the outside of the shell. Mixtures of phosgene and chloropicrin (50-50) have also been used.
The Allies have used a mixture of 80 per cent chloropicrin and 20 per cent stannic chloride (so-called N. C.). This mixture combines the advantages of a gas shell with those of a smoke shell, since the [Pg 149] percentage of stannic chloride is sufficiently high to form a very good cloud. In addition to this, it is believed that the presence of the chloride increases the rate of evaporation of the chloropicrin. It has been claimed that the chloride decreases the amount of decomposition of the chloropicrin upon the bursting of the shell, but careful experiments appear to show that this decomposition is negligible and that the stannic chloride plays no part in it. This mixture was being abandoned at the close of the war.
This N. C. mixture has also been used in Liven’s projectors and in hand grenades. The material is particularly fitted for hand grenades, owing to the low vapor pressure of the chloropicrin, and the consequent absence of pressures even on warm days. As a matter of fact, it was the only filling used for this purpose, though later the stannic chloride was changed, owing to the shortage of tin, to a mixture of silicon and titanium chlorides.
While chloropicrin is sufficiently volatile to keep the strata of air above it thoroughly poisonous, it is still persistent enough to be dangerous after five or six hours.
[Pg 150]
The early idea of gas warfare was that a material, to be of value as a war gas, should have a relatively high vapor pressure. This would, of course, provide a concentration sufficiently high to cause casualties through inhalation of the gas-ladened air. The introduction of “mustard gas” (dichloroethylsulfide) was probably the greatest single development of gas warfare, in that it marked a departure from this early idea, for mustard gas is a liquid boiling at about 220° C., and having a very low vapor pressure. But mustard gas has, in addition, a characteristic property which, combined with its high persistency, makes it the most valuable war gas known at the present time. This peculiar property is its blistering effect upon the skin. Very low concentrations of vapor are capable of “burning” the skin and of producing casualties which require from three weeks to three months for recovery. The combination of these properties removed the necessity for a surprise attack, or the building up of a high concentration in the first few bursts of fire. A few shell, fired over a given area, were sufficient to produce casualties hours and even days afterwards.
Mustard gas, chemically, is dichloroethylsulfide (ClCH₂CH₂)₂S. The name originated with the British Tommy because the crude material first used by the Germans was suggestive of mustard or garlic. Various other names were given the compound, such as “Yellow Cross,” from the shell markings of the Germans; “Yperite,” a name used by the French, because the compound was first used at Ypres; and “blistering gas,” because of its peculiar effect upon the skin. [Pg 151]
It seems probable that an impure form of mustard gas was obtained by Richie (1854) by the action of chlorine upon ethyl sulfide. The substance was first described by Guthrie (1860), who recognized its peculiar and powerful physiological effects. It is interesting in this connection to note that Guthrie studied the effect of ethylene upon the sulfur chlorides, since this reaction was the basis of the method finally adopted by the Allies.
The first careful investigation of mustard gas, which was then only known as dichloroethylsulfide, was carried out by Victor Meyer (1886). Meyer used the reaction between ethylene chlorhydrin and sodium sulfide, with the subsequent treatment with hydrochloric acid. All the German mustard gas used during 1917 and 1918 was apparently made by the use of these reactions, and all the early experimental work of the Allies was in this direction.
Mustard gas was first used as an offensive agent by the Germans on July 12-13, 1917, at Ypres. According to an English report, the physiological properties of mustard gas had been tested by them during the summer of 1916. The Anti-Gas Department put forward the suggestion that it should be used for chemical warfare, but at that time its adoption was not approved. This fact enabled the English to quickly and correctly identify the contents of the first Yellow Cross dud received. It is not true, as reported by the Germans, that the material was first diagnosed as diethylsulfide.
The tactical value of mustard gas was immediately recognized by the Germans and they used tremendous quantities of it. During ten days of the Fall of 1917, it is calculated that over 1,000,000 shell were fired, containing about 2,500 tons of mustard gas. Zanetti states that the British gas casualties during the month following the introduction of mustard gas were almost as numerous as all gas casualties incurred during the previous years of the war. Pope says that the effects of mustard gas as a military weapon were indeed so devastating that by the early autumn of 1917 the technical advisers of the British, French, and [Pg 152] American Governments were occupied upon large scale installations for the manufacture of this material.
The analysis of the first German shell indicated that the mustard gas contained therein had been prepared by the method published by Victor Meyer (1886) and later used by Clark (1912) in England. It was natural, therefore, that attention should be turned to the large scale operation of this method.
The following operations are involved: Ethylene is prepared by the dehydration of ethyl alcohol. The interaction of hypochlorous acid (HClO) and ethylene yields ethylene chlorhydrin, ClCH₂CH₂OH. When this is treated with sodium sulfide, dihydroxyethyl sulfide forms, which, heated with hydrochloric acid, yields dichloroethyl sulfide. Chemically, the reactions may be written as follows:
CH₃CH₂OH = CH₂ : CH₂ + H₂O
CH₂ : CH₂ + HClO = HOCH₂CH₂Cl
2HOCH₂CH₂Cl + Na₂S = (HOCH₂CH₂)₂S + 2NaCl
(HOCH₂CH₂)₂S + 2HCl = (ClCH₂CH₂)₂S + 2H₂O
Without going into the chemistry of this reaction, which is thoroughly discussed by Gomberg[18] (see also German Manufacture), it may be said that this “procedure proved to be unsuitable for large scale production” (Dorsey). As Pope remarks, “That he (the German) should have been able to produce three hundred tons of mustard gas per month by the large scale installation of the purely academic method (of Meyer) constitutes indeed ‘a significant tribute to the potentialities represented by the large German fine chemical factories.’” It is true that a great deal of experimental work was carried out by the Allies on this method, but further study was dropped as soon as the Pope method was discovered.
The first step in advance in the manufacture of mustard gas was the discovery that ethylene would react with sulfur dichloride. While [Pg 153] American chemists were not very successful in their application of this reaction, either in the laboratory or the plant, it was apparently, according to Zanetti, the only method used by the French (the only one of the Allies that manufactured and fired mustard gas). The plant was that of the Société Chimique des Usines du Rhone and was started early in March, 1918, with a production of two to three tons a day. In July it was producing close to twenty tons a day. The plant was being duplicated at the time of the Armistice, so that probably in December, 1918, the production of mustard gas by the dichloride process would have reached about 40 tons. Zanetti points out, however, that the process involved complicated and costly apparatus and required considerable quantities of carbon tetrachloride as a solvent. It is for this reason that the Levinstein process would have been a tremendous gain, had the war continued.
About the end of January, 1918, Pope and Gibson, in a study of the reaction originally used by Guthrie, found that the action of ethylene upon sulfur chloride (S₂Cl₂) at 60° yielded mustard gas and sulfur:
2CH₂ : CH₂ + S₂Cl₂ = (CH₂ClCH₂)₂S + S
The reaction at this temperature caused the separation of sulfur; this occurred after the product stood for some time or immediately if it was treated with moist ammonia gas. While this process was put into commercial operation, both in England and America, it offered considerable difficulty from an operating standpoint. The sulfur would often separate out and block the inlet tubes (ethylene). While it is comparatively easy to remove the mustard gas from the separated sulfur by decantation, a certain amount always remains with the sulfur. It is almost impossible to economically remove this, and its presence adds to the difficulty of removing the sulfur from the reactors; the men engaged in this operation almost always become casualties. [Pg 154]
Fig. 29.—The Levinstein
Reactor
as Installed at Edgewood Arsenal.
It was especially important, therefore, when Green discovered that, if the reaction was carried out at 30°, the sulfur did not settle out but remained in “pseudo solution” in the mustard gas (Pope) or as a loose chemical combination of the monosulfide (mustard gas) with an atom of sulfur (Green). This material has all the physiological activity of the pure monosulfide, while the enormous technical difficulties of handling separated sulfur are entirely obviated by this method of manufacture. To carry out the reaction Levinstein, Ltd., devised the Levinstein “reactor.” The apparatus is shown in Fig. 29. The process consists essentially in bringing together sulfur chloride and very pure ethylene gas in the presence of crude mustard gas as a solvent at a temperature [Pg 155] ranging between 30-35° C. A supply of unchanged monochloride is constantly maintained in the reacting liquid until a sufficiently large batch is built up. Then the sulfur monochloride feed is discontinued and the ethylene feed continued until further absorption ceases. By controlling the ratio of mustard gas to uncombined monochloride, the reaction velocity is so increased that the lower temperature may be used.
The product thus obtained is a pale yellow liquid which deposits no sulfur and requires no further treatment. It is ready for the shell filling plant at once. The obvious advantage of this method led to its adoption in all American plants started for the manufacture of mustard gas (Edgewood, Cleveland and Buffalo).
It was known from the work of certain French chemists that in the presence of such a catalyst as kaolin, ethyl alcohol is dehydrated at an elevated temperature to ethylene. The process as finally developed by American chemists consisted essentially in introducing mixtures of alcohol vapor and steam, in the ratio of one to one by weight, into an 8-inch iron tube with a 3-inch core, in contact with clay at 500-600° C. The use of steam rendered the temperature control more uniform and thus each unit had a greater capacity of a higher grade product. The gaseous products were removed through a water-cooled surface condenser. One unit of this type had a demonstrated capacity of 400 cubic feet per hour of ethylene, between 92 and 95 per cent pure, while the conversion efficiency (alcohol to ethylene) was about 85 per cent. The Edgewood plant consisted of 40 such units. This would have yielded sufficient ethylene to make 40 tons of mustard gas per 24-hour day.
The English procedure consisted in the use of phosphoric acid, absorbed onto coke. An American furnace was designed and built which gave 2,000 cubic feet per hour of ethylene, with a purity of 98 to 99 per cent. This furnace was not used on a large scale, because of the satisfactory nature of the kaolin furnaces. [Pg 156]
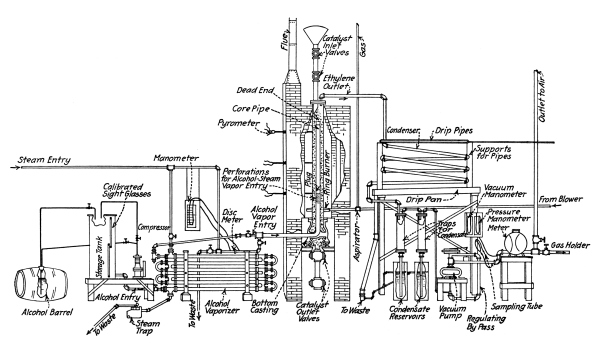
Fig. 30.—Experimental
Installation for the Production
of Ethylene by Kaolin Procedure.
Capacity 400-600 cu. ft. Ethylene per hr.
[Pg 157]
Since chlorine was prepared at Edgewood, it was logical that some of this chlorine should be utilized in the preparation of sulfur chloride. The plant constructed consisted of 30 tanks (78 inches in diameter and 35 feet long), each capable of producing 20,000 pounds of monochloride per day. The tanks are partially filled with sulfur and chlorine passed in. The reaction proceeds rapidly with sufficient heat to keep the sulfur in a molten condition. If the chlorine is passed in too rapidly, the heat generated may be sufficient to boil off the sulfur chloride formed. Hence water pipes are provided so that a supply of cold water may be sprayed upon the tanks, keeping the temperature within the proper limits.
Fig. 31.—Row of Furnaces for the Preparation of Ethylene.
In the manufacture of one ton of mustard gas, about one ton of sulfur chloride and a little less than half a ton of ethylene (12,640 cubic feet) are required. [Pg 158]
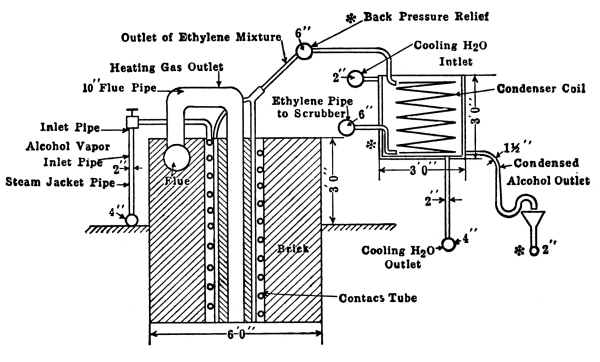
Fig. 32.—Preparation of Ethylene
at
Badische Anilin und Soda Fabrik. 60 units.
“Preparation of Ethylene—The gas was prepared by passing alcohol vapor over aluminum oxide at a temperature of 380° to 400°. The details of the construction of one of the furnaces are given in Figs. 32 and 33. The furnaces were very small and sixty units were needed to furnish the amount of gas required. The tubes containing the catalyzer were made of copper and were heated in a bath of molten potassium nitrate. It was stated that the catalyzer was made according to the directions of Ipatieff, and that its life was from 10 to 20 days. The gas produced was washed in the usual form of scrubber. The yield of ethylene was stated to be about 90 per cent of the theoretical. [Pg 159]
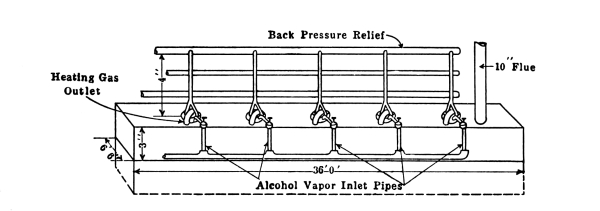
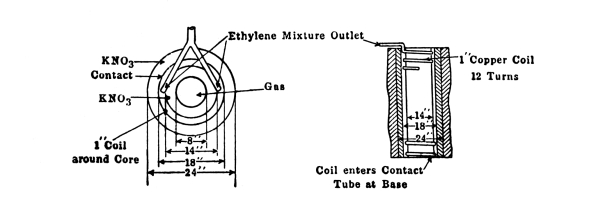
Fig. 33.—Ethylene Production at
Badische
Anilin und Soda Fabrik. 1 unit.
Fig. 34.—Chlorhydrin reaction kettle at
Badische Anilin und Soda Fabrik. 16 units.
“Preparation of Ethylene Chlorhydrin—The reaction was carried out in a cylindrical tank resting on its side. The tank was furnished with a stirrer and was insulated by means of cork in order to prevent the transfer of heat from the atmosphere to the inside. Enough chloride of lime was introduced into the tank to furnish 500 kg. of available chlorine, together with 5 cu. m. of water. At first, about 20 cu. m. of carbon dioxide were led into the mixture, next ethylene, and later carbon dioxide and ethylene simultaneously. The rate of absorption of ethylene was noted and when it slackened, more carbon dioxide was added. Fuller details as to the addition of the two gases were not given as it was stated that it was a matter of judgment on the part of the workman who was carrying out the operation. The reaction should be carried out at as low a temperature as possible, but it was found impossible to work below 5° with the apparatus employed in this factory. The temperature during the reaction varied between 5° and 10°. In order to maintain this temperature, the solution [Pg 160] was constantly pumped from the apparatus through a coil which was cooled by brine. When ethylene was no longer absorbed and there was an excess of carbon dioxide present, the solution was tested for hypochlorous acid. The time required for the introduction of ethylene was between 2 and 3 hrs.
“The contents of the apparatus were passed through a filter press by means of which the calcium carbonate was removed. The solution thus obtained contained from 10 to 12 per cent of ethylene chlorhydrin. It was next distilled with steam and a distillate collected which contained between 18 and 20 per cent of chlorhydrin. The yield of chlorhydrin was from 60 to 80 per cent of that calculated from the ethylene used.
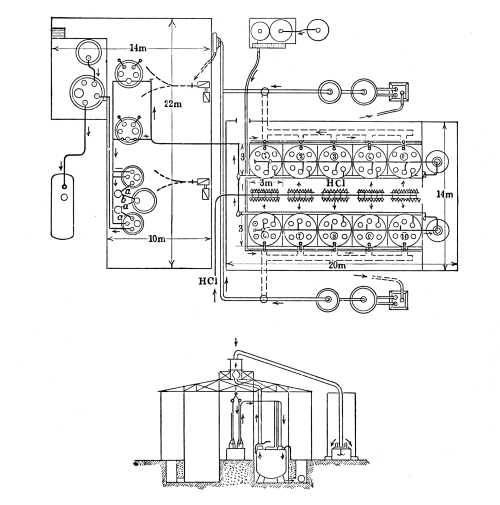
Fig. 35.—Mustard Gas
Manufacture at Leverkusen.
Layout for Chlorination of Thiodiglycol.
“Preparation of Dihydroxyethylsulfide—To prepare the hydroxysulfide, the theoretical quantity of sodium sulfide, either in the form of the anhydrous salt or as crystals, was added to [Pg 161] the 18 to 20 per cent solution of chlorhydrin. After the addition of the sulfide, the mixture was heated to about 90° to 100°. It was then pumped to an evaporator, and heated until all the water was driven off. The glycol was next filtered from the salt which separated, and distilled in a vacuum. The yield of glycol was about 90 per cent of the theoretical, calculated from the chlorhydrin.
“Preparation of Dichlorethylsulfide—The thiodiglycol was taken from the rail to two large storage tanks and thence drawn by vacuum direct to the reaction vessel. Each reaction vessel was placed in a separate cubicle ventilated both from above and below and fitted with glass windows for inspection. The vessels themselves were made of 1¼ in. cast iron and lined with 10 mm. lead. They were 2.5 m. high and 2.8 m. in diameter. These tanks were jacketed so that they could be heated by water and steam, and the reaction was carried out at 50°. The hydrochloric acid coming from the main pipe was passed through sulfuric acid so that the rate could be observed, and passed in by means of 12 glass tubes of about 2 cm. diameter. The rate of flow was maintained at as high a rate as possible to procure absorption. The vapors from the reaction were led from the vessel through a pipe into a collecting room, and then through a scrubber containing charcoal and water, through a separator, and then, finally, into the chimney. These exhaust gases were drawn off by means of a fan which was also connected with the lower part of the chamber in which the reaction vessels were set, so that all the gases had to pass through the scrubber before going to the chimney. When the reaction was completed, the oil was removed by means of a vacuum, induced by a water pump, into a cast iron washing vessel.
“The hydrochloric acid layer was removed to a stoneware receiver, also by vacuum. A glass enabled the operator to avoid drawing oil over with the acid. The pan was fitted with a thermometer to the interior as well as to the jacket. For testing the material during reaction, provision was made for drawing some up by vacuum to a hydrometer contained in a glass funnel. The final test at this point read 126° Tw. Another portion could be drawn up to a test glass and hydrochloric acid passed through it in full view. A float contained in a glass outer tube served to show the level of the liquid in the vessel. The pans in which the operation is carried on, as well as those employed for washing and distilling the product, were of a standard pattern employed in many other operations in the works.
“The washer consisted of a cast iron vessel, lead lined, and was 2.5 m. in diameter, 2 m. deep, and fitted with a dome cover and stirring gear. Lead pipes served for the introduction of sodium carbonate [Pg 162] solution and water. Similar pipes were fitted for drawing these off by means of a vacuum. A manhole on the cover, with a flat top, was fitted with light and sight glasses to which were fitted a small steam coil for keeping them clear. The washed oil is drawn off to a distillation still, which is a cast iron vessel homogeneously lead coated, 1.5 m. in diameter and 2 m. deep, fitted with a lead heating coil and connected through a spiral lead condenser and receiver to a vacuum pump. The water is distilled from the oil at a pressure of from 62 to 70 mm. absolute pressure. When dried, the oil is sent by vacuum to a mixing vessel, similar in most respects to the washing vessel, in which it is mixed with an appointed quantity of solvent, which, in this factory, was usually chlorobenzene but occasionally carbon tetrachloride. The relative quantities varied with the time of year, and instructions were sent from Berlin on this point. Thence the mixture was passed to a storage tank and into tank-wagons.”
The Chemical Warfare Service investigated carefully the three methods (German, French, and English) and finally adopted the Levinstein process. The following discussion is taken from a report originally made during construction, Sept., 1918.
The Levinstein reactor consisted of a jacketed and lead-lined vessel or steel tank, 8 feet 5 inches in diameter and 14 feet tall. The reactor contained 1,400 feet of lead pipe (outside diameter 2⅜ inches), made up into five coils, giving a total cooling surface of 1,200 square feet. The finished charge of such a reactor is 12 tons.
Ethylene was introduced through lead injectors, of which there were 16, each suspended from its own opening in the top and hanging so that the end of the injector tube was 12 inches from the bottom of the reactor. The nozzle of the injector was ³/₁₆ inch outside diameter and ethylene was introduced through it at 40 pounds pressure.
In starting the reaction, enough sulfur chloride was introduced into the reactor to cover the central nozzles. Ethylene was now introduced, and as the reaction proceeded sulfur chloride was added in sufficient quantities to give a high rate of reaction. Brine or cold water was [Pg 163] introduced through the cooling coils and jacket to keep the reacting temperature at 35° C.
When the charge was completed, the ethylene was turned off so that only a small amount bubbled through the nozzles and the charge syphoned off to the settling tank. These were constructed of iron, 8 feet in diameter and 19 feet tall. They were provided with iron coils by which the liquid may be cooled down, or the sulfur, which precipitates in the bottom, melted. The tank was large enough to hold six complete charges of mustard gas and all the sulfur from these charges was allowed to accumulate before removal of the sulfur. The supernatant mustard gas was drawn off from above this sulfur to storage tanks.
Among the factors which influence the reaction are the following:
A temperature of over 60° C. in lead will decompose the product slowly when sulfur chloride is present.
The presence of iron decomposes the product rapidly at a temperature of 50° C. and probably at a considerably lower temperature.
The purity of the product is dependent upon the time of reaction. There is always a slow reaction between the mustard gas and sulfur chloride, and because of this the charge should be completed in 8 hours.
In general the more sulfur that comes out of the solution, the better is the product. Temperature has a marked effect on the separation of sulfur. In order to entirely remove the sulfur from the product it was the custom to increase the temperature at the close of the reaction from 55° to 70° C. This, however, caused plugging of the lines and the reactor.
Dichloroethylsulfide (mustard gas) is a colorless, oily liquid, which has a faint mustard odor. The pure material is said to have an odor very suggestive of that of water cress. While the odor is more or less characteristic, it is possible to have extremely dangerous amounts of the gas in a neighborhood without being detected through its odors. It [Pg 164] still seems to be an open question whether mustard gas paralyzes the sense of smell. One can find opinions on both sides.
Mustard gas boils at 215°-217° C. at atmospheric pressure, so that it is at once seen to be a very persistent gas. It distills without decomposition at this temperature but is best purified by vacuum distillation, or by distillation with steam. A still for the vacuum distillation of mustard gas has been described by Streeter.[20]
Mustard gas melts, when pure, at 13° to 14° C. (The ordinary summer temperature is 20°-25° C.). The ordinary product, as obtained from the “reactor,” melts from 9°-10° C. In order that the product in the shell might be liquid at all temperatures, winter as well as summer, the Germans added from 10 to 30 per cent of chlorobenzene, later using a mixture of chlorobenzene and nitrobenzene and still later pure nitrobenzene. Carbon tetrachloride has also been used as a means of lowering the melting point. Many other mixtures, such as chloropicrin, hydrocyanic acid, bromoacetone, etc., were tested, but were not used. The effect on the melting point of mustard gas is shown in the following table:
Melting point of Mustard gas Mixtures
| Per Cent Added |
Chloropicrin | Chlorobenzene | Carbon Tetrachloride |
|---|---|---|---|
| 0 | 13.4° C. | 13.4° C. | 13.4° C. |
| 10 | 9.8 | 8.4 | 9.8 |
| 20 | 6.3 | 6.4 | 6.6 |
| 30 | 2.6 | -1.0 | 3.1 |
The mustard gas as finally made by the United States contained about 17 to 18 per cent sulfur in solution. The gas was then put in shell and fired without the addition of any solvent. In actual practice this impure product seemed even more powerful in causing casualties than equal quantities of the pure mustard gas. Accordingly no redistilling as originally contemplated was actually carried out. [Pg 165]
The specific gravity of mustard gas at 20° is 1.2741. The solid material has a slightly higher value, being 1.338 at 13°. Its vapor pressure at room temperature is very low; at 20° this value has been found to be about 0.06 mm. of mercury.
Mustard gas is practically insoluble in water, less than 0.1 per cent forming a saturated solution. The reports that a 1 per cent solution could be obtained did not consider the question of hydrolysis. Mustard gas is freely soluble in all the ordinary organic solvents, such as ligroin, alcohol, ether, chloroform, acetic acid, chlorobenzene, etc. In case the solvent is miscible with water, dilution throws out the product as an oil.
Mustard gas is very slowly decomposed by water, owing to its very slight solubility. The products are dihydroxyethylsulfide and hydrochloric acid:
(ClCH₂CH₂)₂S + 2H₂O = (HOCH₂CH₂)₂S + 2HCl
Certain sulfonated oils accelerate the rate of hydrolysis, both by increasing the rate of solution and the solubility of the mustard gas. Alkalies also increase the rate of hydrolysis. Oxidizing agents destroy mustard gas. This reaction was made use of practically in that solid bleaching powder was early introduced as a means of destroying mustard gas in the field. (Fig. 9.)
Chlorinating agents (chlorine, sulfur dichloride, etc.) rapidly transform mustard gas into an inactive (non-blistering) substance. Sulfur dichloride was a valuable reagent in both laboratory and works in “cleaning up” mustard gas. This reaction also explains why the early attempts to prepare mustard gas by the interaction of ethylene and sulfur dichloride were unsuccessful. Mustard gas is probably formed, but is almost immediately chlorinated by the excess of sulfur dichloride. Sulfur chloride on the other hand has no effect on mustard gas. Chloramine-T and Dichloramine-T (the valuable therapeutic agents introduced by Dakin and Carrel for treatment of wounds) also react with [Pg 166] mustard gas. For this reason they were advocated as treatment for mustard gas burns. But as we will see later, they were not altogether successful.
At first the only method of detecting mustard gas was through the sense of smell. It was then believed that concentrations which could not be detected in this way were harmless. Later this proved not to be the case, and more delicate methods had to be devised. In the laboratory and in the field these tests were not very satisfactory, because most of them depended upon the presence of chlorine, and the majority of the war gases contained chlorine or one of the other halogens. The Lantern Test depended upon the accumulation of the halogen upon a copper gauze and the subsequent heating of the gauze in a Bunsen flame. This test could be made to detect one part of mustard gas in ten million parts of air. Another field detector devised by the Chemical Warfare Service consisted in the use of selenious acid. Here again the lack of specificity is apparent, for while certain halogen compounds did not give the test, arsine and organic arsenicals gave a positive reaction and often in a shorter time than mustard gas.
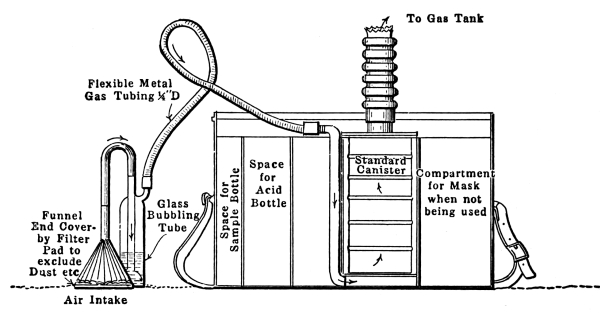
Fig. 36.—Field Detector for Mustard Gas.
The Germans are said to have had plates covered with a yellow composition which had the property of turning black in the presence of [Pg 167] mustard gas. These plates were lowered into the bottom of recently captured trenches and if, after a few minutes, they turned black, the presence of mustard gas was suspected. It is also stated that the characteristic yellow paint on the olive of the mustard gas shell had the same composition, and was useful in detecting leaky shell. According to a deserter’s statement, however, reliance upon this test resulted in casualties in several instances.
A white paint has also been reported which turned red in the presence of mustard gas. This color change was not characteristic, for tests made by our Army showed that other oils (aniline, turpentine, linseed) were found to produce the same effect.
The Chemical Warfare Service was able to develop an enamel and an oil paint which were very sensitive detectors of mustard gas. Both of these were yellow and became dark red in contact with mustard gas. The change was practically instantaneous. The enamel consisted of chrome yellow as pigment mixed with oil scarlet and another dye, and a lacquer vehicle, which is essentially a solution of nitrocellulose in amyl acetate. One gallon of this enamel will cover 946,500 sq. cm., or a surface equivalent to a band 3 cm. wide on 12,500 seven cm. shell.
The paint was composed of a mixture of 50 per cent raw linseed oil and 50 per cent Japan drier, with the above dye mixture added to the required consistency. In contact with liquid mustard gas, this changes to a deep crimson in 4 seconds. Furthermore, in contact with arsenicals, this paint changes to a color varying from deep purple to dark green, the color change being almost instantaneous and very sensitive, even to the vapors of these compounds. Other substances have no effect upon the paint.
For field work, however, nothing was found equal to the trained nose, and it is questionable if any of the mechanical means described will be used in the field.
One of the most interesting phases of mustard gas is its peculiar [Pg 168] physiological action. This has been studied extensively, both as relates to the toxicity and to the skin or blistering effect.
When one considers the high boiling point of mustard gas, and its consequent low vapor pressure, he is likely to conclude that such a substance would be of comparatively little value as a toxic or poison gas. While it is true that an important part of the military value of mustard gas has been because of its vesicant properties, the fact still remains that it is one of our most toxic war gases. The following comparison with a few of the other gases indicates this:
| Mg. per Liter | ||
|---|---|---|
| Mice | Dogs | |
| Mustard gas | 0.2 | 0.05 |
| Phosgene | 0.3 | ··· |
| Hydrocyanic acid | 0.2 | 0.1 |
| Chloropicrin | 1.5 | 0.8 |
| ··· | 3.0 | |
When an animal is exposed to the vapors of mustard gas in high concentration, it subsequently shows a complexity of symptoms, which may be divided into two classes:
(1) The local effects on the eyes, skin and respiratory tract. These are well recognized and consist mainly of conjunctivitis and superficial necrosis of the cornea; hyperemia, œdema and later, necrosis of the skin, leading to a skin lesion of great chronicity; and congestion and necrosis of the epithelial lining of the trachea and bronchi.
(2) The systemic effects due to the absorption of the substance into the blood stream, and its distribution to the various tissues of the body.
The most striking observation about the symptoms of mustard gas poisoning is the latent period which elapses after exposure before any [Pg 169] serious objective or subjective effects are noted. The developments of the effects are then quite slow, unless very high superlethal doses have been inhaled.
At first it was a very serious question whether or not the temporary blindness resulting from mustard gas would not be permanent. Later, as the depth and seriousness of some of the body burns became well known, it was a seven-day wonder that no permanent blindness occurred.
The reason seems to be largely a mechanical one. The constant winking of the eyelids apparently washes the mustard gas off the eyeball and carries it away so that not enough remains to burn to the depth necessary to cause permanent blindness.
Due to the very slight concentrations ordinarily encountered in the field, resulting from a very slow rate of evaporation, the death rate is very low, probably under 1 per cent among the Americans gassed with mustard during the war.
If, on the other hand, the gas be widely and very finely dispersed by a heavy charge of explosive in the shell, the gas is very deadly. In such cases the injured breathe in minute particles of the liquid and thus get hundreds of times the amount of gas that would be inhaled as vapor. This so-called “high explosive mustard gas shell” was a German development in the very last months of the war. Its effects were great enough to make it certain that in the future large numbers of these shell will be used.
The similarity of the symptoms and pathological effects after the inhalation of large amounts of the vapor and those following an injection of an olive oil or water solution of mustard gas led Marshall and his associates to conclude that in high concentrations mustard gas is absorbed through the lungs. A further bit of evidence consists in the isolation of the hydrolysis product, dihydroxyethylsulfide, in the urine of animals poisoned by inhalation of mustard gas. This product is not toxic and is not responsible for the effects of mustard gas. Hydrochloric acid, however, does produce very definite effects upon the animal and may cause death.
From these facts Marshall[21] has proposed the following mechanism of the action of mustard gas: [Pg 170]
“Dichlorethylsulphide is very slightly soluble in water and very freely soluble in organic solvents, or has a high lipoid solubility or partition coefficient. It would, therefore, be expected to penetrate cells very readily. Its rapid powers of penetration are practically proven by its effects upon the skin. Having penetrated within the living cell, it would undoubtedly hydrolyze. The liberation of free hydrochloric acid within the cell would produce serious effects and might account for the actions of dichlorethylsulphide. To summarize, then, the mechanism of the action of dichlorethylsulphide appears to be as follows:
“1. Rapid penetration of the substance into the cell by virtue of its high lipoid solubility.
2. Hydrolysis by the water within the cell, to form hydrochloric acid and dihydroxyethylsulphide.
3. The destructive effect of hydrochloric acid upon some part or mechanism of the cell.
“Although hydrochloric acid does not penetrate cells readily and is easily neutralized by the buffer action of the fluids of the body, we might expect by flooding the body with large quantities of acid to produce some of the characteristic effects of mustard gas. Stimulation of the respiratory center is a well known effect of acid. Convulsions and salivation may be produced by injection of hydrochloric acid and we have been able to produce slowing of the heart by rapid injection of this acid.
“The delayed action of mustard gas might be explained by the formation of some compound with some constituent of the blood. However, blood taken from dogs which had been poisoned with mustard gas and were exhibiting typical symptoms at the time, injected into normal dogs produced no effect. Serum treated in vitro with mustard gas and allowed to stand and then injected into a dog, produced no effect. The fluid which is formed in the vesicle and blebs produced by the application of mustard gas to the skin produces no mustard gas effects.”
In studying the toxicity of mustard gas for dogs, it was observed that a concentration of 0.01 mg. per liter could be tolerated indefinitely. If this value is considered as a threshold value, and subtracted from the toxicity values for varying periods of time, it is found that there is a definite relation between the toxic concentration and the time of exposure. This is expressed by the formula
(C - 0.01)t = K
[Pg 171]
where C is the concentration observed for a given time t. K has the approximate value of 1.7, where t varies between 7.5 and 480 minutes.In addition to its toxicity mustard gas is highly important because of its peculiar irritating effect upon the skin. Its value is seen when we realize that one part in 14,000,000 is capable of causing conjunctivitis of the eye and that one part in 3,000,000 and possibly one part in 5,000,000 will cause a skin burn in a sensitive person on prolonged exposure. According to Warthin, the lesions produced by mustard gas are those of a chemical, not unlike hydrochloric acid, but of much greater intensity. The pathology of these lesions has been carefully studied and fully described by Warthin and Weller in their book on The Pathology of Mustard Gas. Our observations will therefore be confined to certain striking features of the vesicant action of this substance.
Every worker who has worked with mustard gas has noticed that some individuals are much more susceptible to skin burns from this substance than are others. Marshall made a study of 1282 men at Edgewood Arsenal, using a 1 per cent and a 0.01 per cent solution of mustard gas in paraffin oil. A small drop of these solutions was applied to the skin of the forearm of the subject and the arm allowed to remain uncovered for about 10 minutes. The presence or absence of a positive reaction is indicated by the appearance or absence of erythema 24 hours later. The results were as follows:
| 1% | 0.01% | % of Total |
|---|---|---|
| Positive | Positive | 3.3 |
| Positive | Negative | 55.3 |
| Negative | Negative | 41.4 |
[Pg 172]
The test made on 84 negroes gave the following results:
| 1% | 0.01% | % of Total |
|---|---|---|
| Positive | Positive | 0.0 |
| Positive | Negative | 15.0 |
| Negative | Negative | 78.0 |
| Questionable | Negative | 7.0 |
“It is seen from the above tables that negroes as a race, have a much more resistant skin than white men. No negro of the 84 examined reacted to the 0.1 per cent solution, and of course none would react to a more dilute one. About 10 per cent of white men react to the 0.1 per cent solution, while 2 to 3 per cent react to the 0.01 per cent solution or are hypersensitive. About 78 per cent of the negroes fail to react to the 1 per cent solution, while only 20 to 40 per cent of the white race do not show a reaction.”
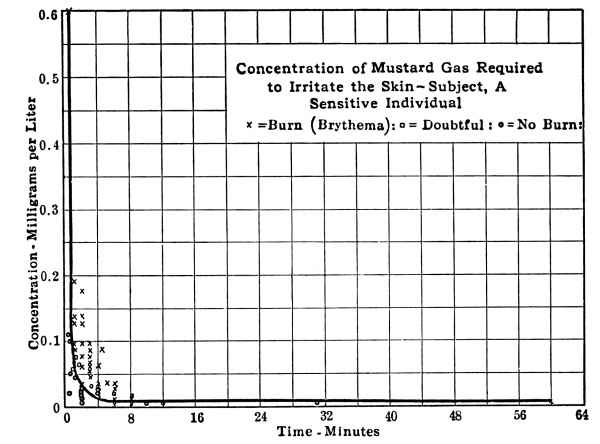
Fig. 37.
The same individual may also show variations in susceptibility and this has also been studied by Marshall.
“The effect of exercise and sweating was investigated. A number of individuals were given vapor burns (one to five minutes exposure) and then exercised until in a profuse sweat, and then the same exposure to vapors made. In all cases the burn produced after exercising [Pg 173] was more severe. Sweating produced by having the subjects place their feet in hot water, produced the same increase in susceptibility. That the moisture on the skin produced by sweating is at least partly, if not entirely, responsible for the increased susceptibility, was shown in the following way: An area of the forearm was kept moist for a few minutes with wet cotton. The sponge was then removed and two vapor tests made, one over the moist area and one over normal, dry skin. In all cases the moist burn was the more severe, in one, producing a blister where the control did not.
“The skin of different areas of the body is undoubtedly somewhat different in its susceptibility. All our tests have been applied to the forearm. The hands are considerably more resistant than the forearm. Tests made by the oil method on the forearm, chest, and back, however, indicate very little difference in susceptibility of these areas. The skin in the neighborhood of old burns has been shown to be more susceptible.
“In general, the same individual does not become more susceptible to skin burns from continued exposure to the vapor. The great number of tests which have been made on the same individual at different times and under the same conditions, indicate a remarkable constancy in reaction. A series of men who were tested at various times during a period of four months, revealed slight changes from time to time in some of the men. No man who originally reacted to only the 1 per cent solution ever reacted to the 0.01, and likewise, no man who originally reacted to the 0.01 ever failed to react to the 0.1 per cent.
“Susceptibility of skin of animals. The paraffin oil test was used on a number of animals and indicated that differences in susceptibility exist in different species and in different individuals of the same species.”
| Species | Number Tested |
Percentage Positive to | ||
|---|---|---|---|---|
| 1 Per Cent |
0.1 Per Cent |
0.01 Per Cent |
||
| Horse | 1 | 100 | 100 | 100 |
| Dog | 91 | 83 | 35 | 0 |
| Goat | 11 | 55 | 36 | 0 |
| Rat | 10 | 30 | 20 | 0 |
| Mouse | 7 | 70 | 14 | 0 |
| Rabbit | 2 | 100 | 0 | 0 |
| Guinea-pig | 12 | 33 | 0 | 0 |
| Monkey | 9 | 22 | 0 | 0 |
[Pg 174] The horse appears to be the most sensitive and the monkey and guinea-pig the most resistant species, while the dog would seem to have a sensitivity as near man as any of the species studied. No animal has yet been found which will give a blister from the application of mustard gas.
Smith, Clowes and Marshall[22] have studied the mechanism of absorption by the skin. They find that it is quite evident that the mustard gas is at first rapidly taken up by some element on, or adjacent to, the surface of the skin and for two to three minutes it may be completely removed, and for ten to fifteen minutes partially removed by prolonged washing With an organic solvent, and to a lesser extent with soap and water.
An interesting phenomenon is observed when the untreated normal skin of one subject is impressed for five minutes upon an area of skin of another subject, which has been exposed previously to the vapors of mustard gas. Under these circumstances both donor and recipient may develop burns (due to the transposition of the poison from one skin to another), the intensity of which will vary according to the circumstances and the respective sensitiveness of the participants. The degree of transposition is most strikingly observed in the intensity of the burn on the donor’s arm. If two similar exposures are made on the arm of a sensitive man, and one of these burns is treated, so to speak, by contact for five minutes, with the skin of a resistant man, the treated burn will be markedly less severe than the control, in some cases being entirely prevented. If, however, the recipient is equally sensitive to or more sensitive than the donor, the burns on the latter will exhibit far less difference. Both treatments may be effected at once, using two recipients, one more, and the other less, resistant than the donor. In such a case the burn brought into contact with the more resistant skin will be the less severe.
Similarly, if a sensitive individual impresses his arm alternately against burns of the same concentration and exposure on a resistant and sensitive man, the recipient receives a more severe burn from the sensitive than from the resistant man. [Pg 175]
This indicates that the skin of a resistant individual exhibits a greater affinity or capacity for mustard gas than that of a sensitive one. There is an actual partition of the gas between the two skins, with an evident tendency to establish an equilibrium in which the larger portion of the gas will remain in that skin which possesses the greater capacity for it.
“A tentative explanation of this phenomenon can be made as follows. A three phase system is involved—the air over the skin surface constitutes the outer phase; some fatty or keratinous elements of the skin, the central phase; and a cellular portion of the skin the inner phase. The central phase is rich in lipoids and poor in water, while the inner phase is rich in water and poor in lipoids. After exposure to the vapors of dichloroethylsulphide the central phase is the absorbing agent and tends to establish equilibrium with the other two phases. On account of the lipoid nature of the central phase no damage is produced here because the compound is not hydrolyzed. On its passage from the central to the inner phase hydrolysis takes place within the cell and damage results when a sufficient concentration of hydrochloric acid is attained. The outer phase is constantly being freed from vapor by diffusion and convection currents, so more and more can evaporate from the central phase. The susceptibility of an individual depends on the relative power of the central phase to hold the poison in an inactive form (not hydrolyzed) and prevent its entry into the inner phase at a sufficient velocity to result in the formation of a toxic concentration. We do not attempt to localize the central or inner phases with any definite structure of the skin. As mustard is known to penetrate the sebaceous ducts the fat here might form one phase and the epithelial lining another.”
As before stated, mustard gas, like most other materials used in war, was discovered in peace. Indeed, Victor Meyer in 1886 worked out fairly completely its dangerous characteristics. Like phosgene and chlorine used before it, the materials for its production were available in considerable quantities through the manufacture of components either for dyes or photographic chemicals.
Mustard gas, besides being highly poisonous, has so many other important qualities as to have given it the designation during the war [Pg 176] of the “king of gases.” That broad distinction it still holds. Its introduction at Ypres, on the night of July 12, 1917, changed completely the whole aspect of gas warfare and to a considerable extent the whole aspect of warfare of every kind. It is highly poisonous, being in that respect one of the most useful of all war gases. It produces no immediate discomfort. It has a considerable delay action. It burns the body inside or out, wherever there is moisture. Eyes, lungs and soft parts of the body are readily attacked. It lingers for two or three days in the warmest weather, while in cold, damp weather it is dangerous for a week or ten days, and in still colder weather may be dangerous for a month or longer whenever the weather warms up sufficient to volatilize the liquid. It is only slowly destroyed in the earth, making digging around shell holes dangerous for weeks and months and in some cases possibly a year or more.
The Germans first used it simply to get casualties and interfere with or break up the threatened heavy attacks by the British on the Ypres salient. While not stopping the inauguration of these attacks in the fall of 1917, the German use of mustard gas was so effective as to delay the beginning of those attacks for at least two weeks and thus gain valuable time for the Germans, besides causing serious casualties with consequent partial break up of companies, regiments and divisions in the English Army.
The German used his mustard gas throughout the fall of 1917 and the winter of 1917 and 1918, as above stated, to produce casualties, to destroy morale, to break up units, and to interfere with operations generally. During that time, however, he developed a more scientific use and when he started his big offensives in March, April, May and June, 1918, he used mustard gas before the battles to cause losses, break up units and destroy morale, and also during the progress of battles to completely neutralize strong points which he felt he did not want to attempt to take by direct assault. Perhaps the most noted case of this was at Armentières in April, when he deluged the city to such an extent that mustard gas is said to have actually run in the streets. So effective was this gassing that not only did the British have to withdraw from the city but the Germans could not enter it for more than two weeks. It, however, enabled the Germans to take the city [Pg 177] with practically no loss of life. There were numerous other cases on a smaller scale where mustard gas was used in the same way.
On account of its persistence it has been generally referred to as a defensive gas and for that purpose it is incomparable. The use of sufficient quantities of mustard gas will almost certainly stop the occupation of areas by the enemy and probably even stop his crossing them. It also enables strong points which it is not desired to attack to be completely neutralized,—that is, made so unhabitable that the area must be evacuated.
A use that was proposed toward the end of the war, and that will undoubtedly be made of the gas in the future, is to have it planted in drums in the ground and exploded when an enemy is attempting to advance. This would be a highly economic way to distribute great quantities of the material at the moment and in the place most heeded. It has even been proposed, and this would seem entirely feasible, to sprinkle certain of these areas with mustard gas by means of sprinklers attached to drums or even tanks mounted on trucks.
Just before the Armistice the German made another development in the use of mustard gas. Instead of the ordinary amount of explosive, which only fairly opened up the shell and allowed the liquid to escape, he filled nearly 30 per cent of the total space of the shell with high explosive. This completely broke up the shell and distributed the greater part of the liquid mustard gas in the form of a fine spray. This spray, when breathed, proved extremely deadly, as might be expected from the fact that when in the form of minute particles one can draw into the lungs in a single breath one hundred times or more the amount that he would get of pure gas.
Since mustard gas has such a delay action and is effective in such small concentrations it can be used very effectively in small calibre guns, as the 75 mm. or 3-inch. Furthermore, since it lasts for two or three days at the very least, a small number of guns can keep a very large area neutralized with the gas. With phosgene and similar non-persistent gases that volatilize almost completely upon the burst of the shell it is necessary to build up a high concentration immediately. The exact opposite is true of mustard gas. Mustard gas can be fired very slowly with the certain knowledge that all shells fired [Pg 178] at one moment will be effective when the next is fired, though twelve hours or more may intervene between the first and last firing. Thus, while with phosgene a large number of guns are needed for a gas attack, with mustard gas the number can be reduced to one-tenth or even less. Mustard gas may be in the future and has been in the past used safely in hand grenades because of its very low vapor tension, whereby the pressure at ordinary temperatures is exceedingly low. This has an important bearing on cylinders and other containers for shipping mustard gas, that is, they need be only strong enough to be safe against handling and not to withstand the high pressure encountered with phosgene or chlorine cylinders.
In the future, mustard gas will be used in all the ways above stated and undoubtedly in many more. It can be fired in large quantities upon strong points to force their evacuation. It can be fired on the flank of attacking armies for protection against counter-attacks. It can be fired against the enemy artillery at all times to silence them and stop their firing. It was thus used by the Americans in the Argonne against the enemy on the east bank of the Meuse River, this river separating the American and German armies. It was extremely effective in stopping the enemy’s artillery. The high explosive mustard gas shell, not only because of its persistency but because of its quick deadliness, can be fired singly and be depended upon to do its work wherever there be men or animals. One of the greatest uses will be by simple sprinkling from aeroplanes.
The future will see mustard gas used at nearly all times with a certain quantity of a powerful lachrymator or tear gas. This is for the reasons, as stated in the beginning, that mustard gas causes no immediate discomfort and has no objectionable smell. Accordingly, if the battle be critical, men may continue to fight from four to eight hours in a mustard gas atmosphere without masks. It is true the casualties will be high with a high death rate. Nevertheless, this period of time might enable the artillery to do such effective work as to completely stop an attack. If, however, at the instant mustard gas firing is begun a number of powerful lachrymatory shells are sent over, the immediate wearing of the mask is forced. The enemy is then subject [Pg 179] to all the burning effects of mustard gas as well as the discomfort of long wearing of the mask. [Pg 180]
It may confidently be expected that further developments in the use of mustard gas will be made, as well as further developments in methods of throwing it upon the enemy or of bursting shell containing it in his midst.
Since arsenic is well known as an insecticide in the form of lead arsenate, arsenic acid etc., and in pharmacy, specially in the form of salvarsan and neosalvarsan, it is not surprising that the Germans should have endeavored to discover an arsenic derivative which would be of value from the point of view of chemical warfare. Very early in the war persistent rumors were circulated that the Germans were to use arsine. These rumors led to the use of sodium permanganate in the canister, but as far as is known, no arsine was actually used. Another suggestion which received considerable attention from American workers was the use of arsenides, which might decompose under the influence of the atmospheric moisture with the liberation of arsine. Calculation of the amount of arsenide necessary to establish a lethal concentration of arsine showed, however, that there was no possibility of using the material on the field.
Because of the use of arsenic trichloride in the manufacture of organic arsenic compounds, a method of preparation was developed from arsenic trioxide and sulfur chloride or hydrogen chloride. It was also shown experimentally that the phosgene of the tail gas of phosgene plants might be converted into arsenic trichloride by reaction with arsenic trioxide. Charcoal is the catalyzer of this reaction.
Arsenic trichloride is also of interest because it was one of the constituents of the mixture vincennite, early used by the French. This was a mixture of hydrocyanic acid, stannic chloride, arsenic trichloride and chloroform. While extensively used at first, it was gradually replaced by phosgene.
Arsenic triflouride was also prepared by the action of sulfuric acid [Pg 181] upon a mixture of calcium flouride and arsenic trioxide. The compound is very easily decomposed by the moisture of the air, and furthermore is not very toxic.
Organic arsenic derivatives are the most important compounds from the military point of view. The first substance used was diphylchloroarsine, a white solid, which readily penetrated the canister and caused sneezing. This was used alone, and in solution in phenyl dichloroarsine. Later methyl and ethyl dichloroarsines were introduced.
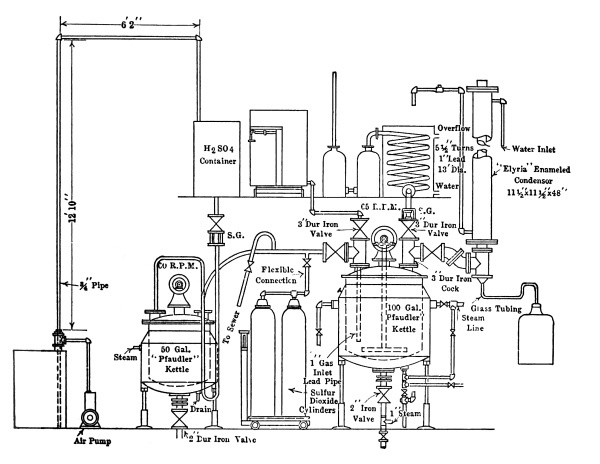
Fig. 38.—Apparatus for
the Manufacture of
Methyldichloroarsine.
The Germans apparently used ethyldichloroarsine because they had no suitable method for the preparation of methyl dichloroarsine, which is a more satisfactory material. The Chemical Warfare Service developed the following method of preparation of the methyl derivative. Sodium arsenite (Na₃AsO₃) is prepared by dissolving arsenic trioxide in sodium hydroxide solution. The action of methyl sulfate at 850 C. gives [Pg 182] disodium methyl arsenite, Na₂CH₃AsO₃. Sulfur dioxide reduces the arsenite to methyl arsine oxide, CH₃AsO, which is then reacted with hydrochloric acid to give methyl dichloroarsine. The final product is distilled from the mixture and condensed. This material costs from two to two and a half dollars per pound for chemicals (war prices).
Methyldichloroarsine is a colorless liquid of powerful burning odor, which boils at 132° C. It is somewhat soluble in water and is soluble in organic solvents. The specific gravity is 1.838 at 20° C. The vapor pressure at 25° was found to be 10.83 mm. mercury. Not only is the material toxic but it has remarkable vesicant properties, comparing favorably with mustard gas in this respect.
Ethyldichloroarsine, which was used by the Germans, was prepared by the method given above, using ethyl sulfate, but the yield was never over 20 per cent. In general this has properties similar to the methyl derivative.
The best known of the arsenicals, however, is diphenylchloroarsine or sneezing gas. Although this is an old compound (having been prepared by German chemists in 1885), there was no method for its preparation on a large scale when, first introduced into chemical warfare. It was finally discovered that the interaction of triphenyl arsine with arsenic trichloride was fairly satisfactory and a plant was erected for its manufacture.
When pure, diphenylchloroarsine is a colorless solid, melting at 44°. Because of this, it was always used in solution in a toxic gas or in a shell which contained a large amount of explosive so that on the opening of the shell the material would be finely divided and scattered over a wide territory.
Its value lay in the fact that the fine particles readily penetrated the ordinary mask and caused the irritation of the nose and throat, which resulted in sneezing. This necessitated the perfection of special smoke filters to remove the particles, after which the other toxic materials were removed by the absorbent in the canister. [Pg 183]
It causes sneezing and severe burning sensations in the nose, throat and lungs in concentrations as slight as 1 part in 10 million. In higher concentrations, say 1 in 200 to 500 thousand it causes severe vomiting. While neither of these effects are dangerous or very lasting, still higher concentrations are serious, as in equal concentrations diphenylchloroarsine is more poisonous than phosgene.
Various other arsenical chemicals were developed in the laboratory, but with one or two exceptions they were not as valuable as diphenylchloroarsine and methyldichloroarsine and were therefore discarded.
“This substance (Blue Cross) was a famous gas of the Germans and was made in large quantities. The method used by the Germans was different from the one worked out by the Allies, and on account of the fact that the German method could be carried out without specially designed apparatus and required as raw materials substances readily obtainable, it was probably preferable. It is doubtful, however, whether the Allies would have made this gas, for as the result of its use no fatalities were reported. The German process consisted in preparing phenylarsenic acid by condensing benzene diazonium chloride with sodium arsenite. The acid was next reduced by sulfur dioxide to phenylarsenous acid, which was, in turn, condensed with the diazonium compound to form diphenylarsenic acid. This acid was reduced to diphenylarsenous oxide, which with hydrochloric acid yielded diphenylchloroarsine. The chemical equations for the reactions will make clearer the steps involved.
| C₆H₅N₂Cl + Na₃AsO₃ | = | C₆H₅AsO₃Na₂ + NaCl + N₂ |
| C₆H₅AsO₃Na₂ + 2HCl | = | C₆H₅AsO₃H₂ + 2NaCl |
| C₆H₅AsO₃H₂ + SO₂+H₂O | = | C₆H₅AsO₂H₂ + H₂SO₄ |
| C₆H₅N₂Cl + C₆H₅AsO₂Na₂ | = | (C₆H₅)₂AsO₂Na + NaCl + N₂ |
| (C₆H₅)₂AsO₂Na + HCl | = | (C₆H₅)₂AsO₂H + NaCl |
| 2(C₆H₅)₂AsO₂H + 2SO₂ + H₂O | = | [(C₆H₅)₂As]₂O + 2H₂SO₄ |
| [(C₆H₅)₂As]₂O + 2HCl | = | 2(C₆H₅)₂AsCl + H₂O. |
“The entire process was carried out at Höchst. The method used at Höchst was as follows: In preparing the diazonium solution, 3 kg.-mols [Pg 184] of aniline were dissolved in 3000 liters of water and the theoretical quantity of hydrochloric acid. The temperature of the solution was reduced to between 0° and 5° and the theoretical amount of sodium nitrite added. The reaction was carried out in a wooden tank of the usual form for the preparation of diazonium compounds. A solution of sodium arsenite was prepared which contained 20 per cent excess of oxide over that required to react with the aniline used. The arsenous oxide was dissolved in sodium carbonate, care being taken to have enough of the alkali present to neutralize all of the acid present in the solution of the diazonium salt. To the solution of the sodium arsenite were added 20 kg. of copper sulfate dissolved in water, this being the amount required when 3 kg.-mols of aniline are used. The solution of the diazonium compound was allowed to flow slowly into the solution of the arsenite while the temperature was maintained at 15°. The mixture was constantly stirred during the addition which requires about 3 hrs. After the reaction was complete, the material was passed through a filter press in order to remove the coupling agent and the tar which had been formed. Hydrochloric acid was next added to the clear solution to precipitate phenylarsenic acid, the last portions of which were removed by the addition of salt.
“The phenylarsenic acid was next reduced to phenylarsenous acid by means of a solution of sodium bisulfite, about 20 per cent excess of the latter over the theoretical amount being used. For 100 parts of arsenic acid, 400 parts of solution were used. The reaction was carried out in a wooden vessel and the mixture stirred during the entire operation. A temperature of 80° was maintained by means of a steam coil. Phenylarsenous acid separated as an oil. The aqueous solution was decanted from the oil, which was dissolved in a solution of sodium hydroxide, 40° Bé. The solution of the sodium salt of phenylarsenous acid was treated with water so that the resulting solution had a volume of 6 cu. m. when 3 kg.-mols of the salt were present. Ice was next added to reduce the temperature to 15° and a solution of benzene diazonium chloride, prepared in the manner described for the first operation, was slowly added. After the coupling, diphenylarsenic acid was precipitated by means of hydrochloric acid. The acid was removed by means of a filter press and dissolved in hydrochloric acid, 20° Bé. For one part of diphenylarsenic acid, 3 parts of hydrochloric acid were used. Into this solution was passed 5 per cent excess of sulfur dioxide over that required for the reduction. The sulfur dioxide used was obtained from cylinders which contained it in liquid condition.
“The reduction was carried out in an iron tank lined with tiles [Pg 185] and a temperature of 80° was maintained. About 8 hrs. were required for the reaction. The diphenylarsenic acid on reduction by the sulfur dioxide was converted into diphenylarsenous oxide which, in the presence of the hydrochloric acid, was converted into diphenylchloroarsine, which separated as an oil. The oil was next removed and heated in the best vacuum obtainable until it was dry and free from hydrochloric acid. The compound melted at 34°. It was placed in iron tanks for shipment. The yield of diphenylchloroarsine calculated from the aniline used was from 25 to 30 per cent of the theoretical. No marked trouble was observed in handling the materials and no serious poisoning cases were reported.
“This compound was prepared by treating diphenylchloroarsine with a saturated aqueous solution of potassium or sodium cyanide.
(C₆H₅)₂AsCl + NaCN = (C₆H₅)₂AsCN + NaCl.
Five per cent excess of the alkaline cyanide was used. The reaction was carried out at 60° with vigorous stirring. The yield was nearly theoretical.
“This compound was prepared at Höchst from ethylarsenous oxide which was obtained from the Badische Anilin und Soda Fabrik.
“Preparation of Ethylarsenous Oxide—The compound was prepared by treating sodium arsenite with ethyl chloride under pressure. The resulting sodium salt of ethylarsenic acid was converted into the free acid and reduced by sulfur dioxide. The ethylarsenous acid formed in this way lost water and was thereby transformed into ethylarsenous oxide. The reactions involved are as follows:
| C₂H₅Cl + Na₃AsO₃ | = | C₂H₅AsO₃Na₂ + NaCl |
| C₂H₅AsO₃Na₂ + 2 HCl | = | C₂H₅AsO₃H₂ + 2 NaCl |
| C₂H₅AsO₃H₂ + SO₂ + H₂O | = | C₂H₅AsO₂H₂ + H₂SO₄ |
| 2C₂H₅AsO₂H₂ | = | (C₂H₅As)₂O + H₂O. |
“The ethyl chloride used in the preparation was in part made in this factory, and in part received from other sources. As ethyl chloride is an important product used in peace time, it is not, therefore, essentially a war product and its preparation was not described.
“In preparing the solution of sodium arsenite, one molecular weight of arsenous oxide was dissolved in a solution containing 8 molecular [Pg 186] weights of sodium hydroxide. The solution of the base was prepared from a 50 per cent solution of sodium hydroxide to which enough solid alkali was added to make the solution a 55 per cent one. In one operation 660 kg. of arsenous oxide were used. For 100 parts of arsenous oxide, 130 parts of ethyl chloride were used, this being the theoretical amount of the latter.
“The reaction was carried out in a steel autoclave of about 300 liters capacity. The temperature was maintained at between 90° and 95°. The ethyl chloride was pumped in, in 3 or 4 portions, and the pressure in the autoclave was kept at from 10 to 15 atmospheres. The several portions of ethyl chloride were introduced at intervals of about 1½ hrs. During the entire reaction, the contents of the autoclave were vigorously stirred. After all the ethyl chloride had been added, the material was stirred from 12 to 16 hrs., at the end of which time the pressure had fallen to about 6 atmospheres. The excess of ethyl chloride and the alcohol formed in the reaction were next distilled off. At this point a sample of the solution was drawn off for testing. This was done by determining the amount of arsenite present in the solution. If not more than 20 per cent of sodium arsenite had not reacted, the preparation was considered satisfactory. Water was then added to the contents of the autoclave in sufficient amount to dissolve the solid material. The product was next drawn over into a tank and neutralized with sulfuric acid. It was then treated with sulfur dioxide gas until there was an excess of the latter present. The mixture was then heated to about 70° when the ethylarsenous oxide precipitated as a heavy oil. This was readily separated and shipped without further purification. The yield of ethylarsenous oxide, from arsenic oxide, was from 80 to 82 per cent of a product which contained about 93 per cent of pure ethylarsenous oxide.
“Preparation of Ethyldichloroarsine—The compound was prepared by treating ethylarsenous oxide with hydrochloric acid. The reaction is as follows:
C₂H₅AsO + 2HCl = C₂H₅AsCl + H₂O.
The operation was carried out in an iron kettle lined with lead, which was cooled externally by means of water and which was furnished with a lead covered stirrer. To the kettle, which contained from 500 to 1000 kg. of hydrochloric acid left over from the previous operation, were added 4000 kg. of ethylarsenous oxide. The gaseous hydrochloric acid was next led in. The kettle was kept under slightly diminished pressure in order to assist in the introduction of hydrochloric acid. The [Pg 187] temperature during the reaction must not rise above 95°. When the hydrochloric acid was no longer absorbed and was contained in appreciable quantities in the issuing gases, the operation was stopped. This usually occurred at the end of from one to two days. The product of the reaction was drawn off by means of a water pump and heated in a vacuum until drops of oil passed over. The residue was passed over to a measuring tank and finally to tank-wagons made of iron. The yield of the product was practically the theoretical.
On account of the volatility of the compound and its poisonous character, the apparatus in which it was prepared was surrounded by an octagonal box, the sides of which were fitted with glass windows. Through this chamber a constant supply of air was drawn. This was led into a chimney where the poisonous vapors were burned. The gases given off during the distillation of the product were passed through a water scrubber.”
The one arsenical which created the most discussion during the War, and about which many wild stories were circulated, was “Lewisite,” or as the press called it, “Methyl.” Its discovery and perfection illustrate the possibilities of research as applied to Chemical Warfare, and points to the need of a permanent organization to carry on such work when the pressure of the situation does not demand such immediate results.
The reaction of ethylene and sulfur chloride, which led to the preparation of mustard gas, naturally led the organic chemists to investigate the reaction of this gas and other unsaturated hydrocarbons, such as acetylene, upon other inorganic chlorides, such as arsenic, antimony and tin. There was little absorption of the gas, either at atmospheric or higher pressures, and upon distilling the reaction product, most of the gas was evolved, showing that no chemical reaction had taken place. However, when a catalyser, in the form of aluminium chloride, was added, Capt. Lewis found that there was a vigorous reaction and that a highly vesicant product was formed. The possibilities of this compound were immediately recognized and the greatest secrecy was maintained regarding all the details of preparation and of the properties of this new product. At the close of [Pg 188] the War, this was considered one of the most valuable of Chemical Warfare secrets, and therefore publication of the reactions involved were withheld. Unfortunately or otherwise, the British later decided to release the material for publication, and details may be found in an article by Green and Price in the Journal of the Chemical Society for April, 1921. It must be emphasized that the credit for this work belongs, not to these authors, but to Capt. W. Lee Lewis and the men who worked with him at the Catholic University branch of the American University Division (the Research Division of the C. W. S.).
On a laboratory scale, acetylene is bubbled through a mixture of 440 grams of anhydrous arsenic trichloride and 300 grams of anhydrous aluminium chloride. Absorption is rapid and much heat is developed. After six hours, about 100 grams of acetylene is absorbed. The reaction product was dark colored and viscid, and had developed a very powerful odor, suggestive of pelargoniums. Attempts to distill this product always led to violent explosions. (It may be stated here that Lewis was able to perfect a method of distillation and separation of the products formed, so that pure materials could be obtained, with little or no danger of explosion.) The English chemists therefore decomposed the product with ice-cold hydrochloric acid solution of constant boiling point (this suggestion was the result of work done by Lewis). The resulting oil was then distilled in a current of vapor obtained from constant boiling hydrochloric acid and finally fractionated into three parts.
The first product obtained consist in the addition of one acetylene to the arsenic trichloride molecule, and, chemically, is chlorovinyldichloroarsine, CHCl: CH·AsCl₂, a colorless or faintly yellow liquid, boiling at 93° at a pressure of 26 mm. A small quantity, even in very dilute solution, applied to the skin causes painful blistering, its virulence in this respect approaching that of mustard gas. It is more valuable than mustard gas, however, in that it is absorbed through the skin, and as stated on page 23, three drops, placed on the abdomen of a rat, will cause death in from one to three hours. It is also a very powerful respiratory irritant, the mucous membrane of the nose being attacked and violent sneezing induced. More [Pg 189] prolonged exposure leads to severe pain in the throat and chest.
The second fraction (β, β′-dichlorodivinylchloroarsine) is a product resulting from the addition of two acetylene molecules to one arsenic trichloride, and boils at 130° to 133° at 26 mm. It is much less powerful as a vesicant than chlorovinyldichloroarsine, but its irritant properties on the respiratory system are much more intense.
The third fraction, β, β′, β″-trichlorotrivinylarsine, (CHCl: CH)₃As, is a colorless liquid, boiling at 151° to 155° at 28 mm., which solidifies at 3° to 4°. It is neither a strong vesicating agent nor a powerful respiratory irritant. At the same time, its odor is pungent and most unpleasant and it induces violent sneezing.
[Pg 190]
Carbon monoxide, because of its cheapness, accessibility and ease of manufacture, has been frequently considered as a possible war gas. Actually, it appears never to have been used intentionally for such purposes. There are several reasons for this. First, its temperature of liquefaction at atmospheric pressure is -139° C. This means too high a pressure in the bomb or shell at ordinary temperatures. Secondly, the weight of carbon monoxide is only slightly less than that of air, which keeps it from rolling into depressions, dugouts and trenches, as in the case of ordinary gases, and also permits of its rather rapid rise and dissipation into the surrounding atmosphere. A third reason is its comparatively low toxic value, which is only about one-fifth that of phosgene. However, as it can be breathed without any discomfort, and as it has some delay action, its lack of poisonous properties would not seriously militate against its use were it not for the other reasons given.
It is, nevertheless, a source of serious danger both in marine and land warfare. Defective ventilation in the boiler rooms of ships and fires below decks, both in and out of action, are especially dangerous because of the carbon monoxide which is produced. In one of the naval engagements between the Germans and the English, defective high explosive shell, after penetrating into enclosed portions of the ship, evolved large quantities of carbon monoxide and thus killed some hundreds of men. On shore, machine gun fire in enclosed spaces, such as pill boxes, and in tanks, liberates relatively large quantities of carbon monoxide. Similarly, in mining and sapping work, the carbon monoxide liberated by the detonation of high explosives constitutes one of the most serious of the difficulties connected with this work and [Pg 191] necessitated elaborate equipment and extensive military training in mine rescue work.
The removal of carbon monoxide from the air is difficult because of its physical and chemical properties. Its low boiling point and critical temperature makes adequate adsorption at ordinary temperatures by the use of an active absorbent out of the question. Its known insolubility in all solvents similarly precludes its removal by physical absorption.
After extensive investigation two absorbents have been found.[24] The first of these consists in a mixture of iodine pentoxide and fuming sulfuric acid, with pumice stone as a carrier. Using a layer 10 cm. deep and passing a 1 per cent carbon monoxide air mixture at the rate of 500 cc. per minute per sq. cm. cross section, a 100%-90% removal of the gas could be secured for two hours at room temperature and almost as long at 0° C. The reaction is not instantaneous, and a brief induction period always occurs. This may be reduced to a minimum by the addition of a little iodine to the original mixture.
The sulfur trioxide given off is very irritating to the lungs, but by the use of a layer of active charcoal beyond the carbon monoxide absorbent, this disadvantage was almost completely eliminated. However, sulfur dioxide is slowly formed as a result of this adsorption and after prolonged standing or long-continued use of the canister at a high rate of gas flow gives serious trouble.
Considerable heat is given off in the reaction and a cooling attachment was required. The most satisfactory device was a metal box filled with fused sodium thiosulfate pentahydrate, which absorbed a very considerable amount of the heat.
Still a further disadvantage was the fact that the adsorbents became spent by use, even in the absence of carbon monoxide, since it absorbed enough moisture from the air of average humidity in several hours, to destroy its activity.
The difficulties mentioned were so troublesome that this absorbent was finally supplanted by the more satisfactory oxide absorbent described below. [Pg 192]
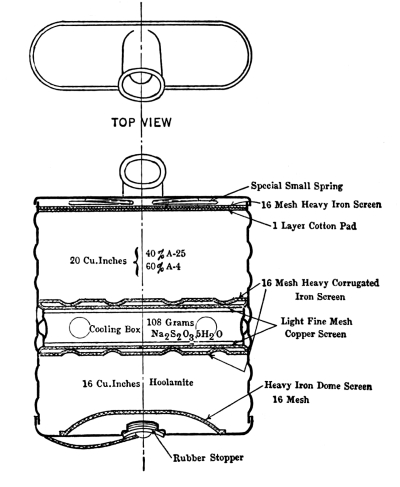
Fig. 39.—Diagram of Carbon Monoxide Canister, CMA3.
The metallic oxide mixture was the direct result of an observation that specially precipitated copper oxide with 1 per cent silver oxide was an efficient catalyst for the oxidation of arsine by oxygen. After a study of various oxide mixtures, it was found that a mixture of manganese dioxide and silver oxide, or a three component system containing cobaltic oxide, manganese dioxide and silver oxide in the proportion of 20:34:46 catalyzed the reaction of carbon monoxide at room temperature. The studies were extended and it was soon found that the best catalysts contained active manganese dioxide as the chief constituent. This was prepared by the reaction between potassium permanganate and anhydrous manganese sulfate in the presence of fairly concentrated sulfuric acid. It also developed that the minimum silver oxide content decreased progressively as the number of components increased from 2 to 4. The standard catalyst (Hopcalite) finally adopted for production consisted of 50 per cent manganese dioxide, 30 per cent copper oxide, 15 per cent cobaltic oxide and 5 per cent silver oxide. The mixture was prepared by [Pg 193] precipitating and washing the first three oxides separately, and then precipitating the silver oxide in the mixed sludge. After washing, this sludge was run through a filter press, kneaded in a machine, the cake dried and ground to size. While it is not difficult to obtain a product which is catalytically active, it requires a vigorous control of all the conditions and operations to assure a product at once active, hard, dense and resistant as possible to the deleterious action of water vapor.
Fig. 40.—Tanks and Press
for Small Scale Manufacture
of Carbon Monoxide Absorbent.
Hopcalite acts catalytically and therefore only a layer sufficiently deep to insure close contact of all the air with the catalyst is needed. One and a half inches (310 gm.) were found ample for this purpose.
The normal activity of Hopcalite requires a dry gas mixture. This was secured by placing a three-inch layer of dry granular calcium chloride at the inlet side of the canister.
Because of the evolution of heat, a cooling arrangement was also used in the Hopcalite canisters.
The life of this canister was the same irrespective of whether its use [Pg 194] was continuous or intermittent. The higher the temperature the longer the life because Hopcalite is less sensitive to water vapors at higher temperatures. Since, if the effluent air was sufficiently dried, the Hopcalite should function indefinitely against any concentration of carbon monoxide, the life of the canister is limited solely by the life of the drier. Therefore the net gain in weight is a sure criterion of its condition. After many tests it was determined that any canister which had gained more than 35 grams above its original weight should be withdrawn. The canisters, at the time of breakdown, showed a gain in weight varying between 42 and 71 grams, with a average of 54 grams. It is really, therefore, the actual humidity of the air in which the canister is used that determines its life.

Fig. 41.—Navy Head Mask and Canister.
[Pg 195]
While in ordinary warfare the best defense against any implement of war is a vigorous offense with the same weapon, Chemical Warfare presents a new point of view. Here it is very important to make use of all defensive measures against attack. Because of the nature of the materials used, it has been found possible to furnish, not only general protection, but also continuous protection during the time the gas is present.
The first consideration in the protection of troops against a gas attack is the provision of an efficient individual protective appliance for each soldier. The gas attack of April 22, 1915 found the Allies entirely unprepared and unprotected against poisonous gas. While a few of the men had the presence of mind to protect themselves by covering their faces with wet cloths, the majority of them became casualties. Immediately steps were taken to improvise protective devices among which were gags, made with rags soaked in water or washing soda solution, handkerchiefs filled with moist earth, etc. One suggestion was to use bottles with the bottom knocked off and filled with moist earth. The breath was to be taken in through the bottle and let out through the nose; but as bottles were scarce and few of them survived the attempt to get the bottom broken off, the idea was of no value.
The first masks were made by the women of England in response to the appeal by Lord Kitchener; they consisted of cotton wool wrapped in muslin or veiling and were to be kept moist with water, soda solution or hypo.
The Black Veiling Respirator. The first form of the English mask [Pg 196] is known as the Black Veiling respirator and consisted of cotton waste enclosed in a length of black veiling. The waste was soaked in a solution of:
| Sodium thiosulphate | 10 | lbs. |
| Washing soda | 2.5 | lbs. |
| Glycerine | 2 | lbs. |
| Water | 2 | gals. |
The glycerine was put in to keep the respirator moist, thus obviating the need for dipping before use.
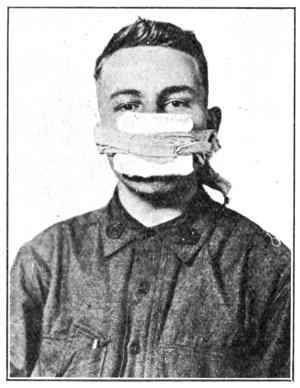
Fig. 42.—Early Gas Protection.
The respirator was adjusted over the mouth and nose, the cotton waste being molded to the shape of the face and the upper edge of the veiling pulled up so as to protect the eyes. These respirators were used in the attacks of May 10th and 12th, 1915 and were reasonably efficient against the low concentration of chlorine then used; they were difficult to fit exactly to the face, which resulted in leakage. The cotton waste often became lumpy and had to be shredded out or discarded.
The Hypo Helmet. The next development of the British protection was the so-called Hypo helmet. This is said to have resulted from the suggestion of a Canadian sergeant that he had seen a German pulling a bag over his head during a gas attack. It consisted of a flannel bag [Pg 197] soaked in the same solution as was used for the veiling respirator and was fitted with a pane of mica as a window. The helmet was tucked down inside the jacket which was then buttoned up tightly around the neck. As may be seen from Figure 43, this would not prove very satisfactory with the American type of uniform.
This helmet had many advantages over the veiling respirator but the window often became cracked or broken from the rough treatment in the trenches. Later the mica was replaced by celluloid and still later by glass eyepieces set in metal rings. These were very effective against chlorine in the field.
The P and PH Helmets. During the summer of 1915 it became evident that phosgene-chlorine mixtures would be used in gas attacks and it was therefore necessary to provide protection against this. The hypo helmet, which offered no protection against phosgene, was soaked in an alkaline solution of sodium phenolate (carbolic acid) containing glycerine, and with this new form of impregnation was called the P helmet. It protected against 300 parts of phosgene in a million of air. Since this solution attacks flannel, two layers of flannelette were used. The helmet was further improved by the addition of an expiratory valve, partly to prevent the man from breathing any of his own breath over again and partly to prevent the deterioration of the alkali of the mask by the carbon dioxide of the expired air.
The protection was later further increased by the addition of hexamethylenetetramine, and this mask is known as the PH helmet. This increased the protection to 1,000 p.p.m.
The early types of helmet offered no protection against lachrymators. For this purpose goggles were used, the later types of which had glass eyepieces and were fitted around the eyes by means of rubber sponge. While intended for use only after a lachrymatory bombardment, the troops frequently used them during and after an ordinary gas attack when the mask should have been worn. Consequently they were withdrawn.
The PH helmet was unsatisfactory because of the following reasons:
[Pg 198]

Fig. 43.—Method of Wearing
the P. H. Helmet
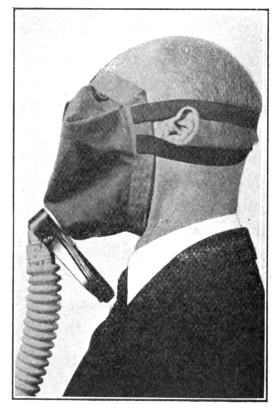
Fig. 44.—Early Type of Standard
(British) Box Respirator (S. B. R.)
Box Respirator. The increasing concentration of gas from cylinder attacks and the introduction of shell, with such gases as chloropicrin and superpalite, led, early in 1916, to very definite and constructive efforts on the part of the British to increase the protection offered by the mask. The result was a “polyvalent” respirator of the canister type (the Standard Box Respirator). This mask was probably the result of experience with oxygen apparatus in mine rescue work. The lines on which this canister was modeled involved the use of a canister filled with highly sensitive absorbent charcoal mixed with or alternating in layers with oxidizing granules of alkaline permanganate. It was the result of innumerable experiments, partly conducted in France but mostly in England under the direction of the [Pg 199] late Lieut. Col. Harrison, who was almost entirely responsible for the wonderful production of this respirator.
The respirator (Figure 44) consisted of the canister mentioned above, which is attached by a flexible tube to a facepiece or mask. The facepiece is made of rubberized fabric and fits the face so that there is little or no leakage. This is secured by means of tape and elastic bands which fit over the head. The nose is closed by means of clips, which are wire springs with rubbered jaws covered with gauze (Fig. 45). Breathing is done through a mouthpiece of rubber; the teeth close on the rubber tabs and the rubber flange lies between the teeth and the lips. The expired air finds exit through a rubber flutter valve in an angle tube just outside the mask. This arrangement furnishes a double line of protection; if the face piece is punctured or torn, gas-containing air cannot be breathed as long as the noseclip and mouthpiece are in position.
The early English canister was packed with 675 cc. of 8-14 mesh war gas mixture, 40 per cent of which was wood charcoal and 60 per cent reddish brown soda-lime granules. The metal dome at the bottom of the can was covered with a thin film of cotton. At two-thirds of the distance to the top was placed a paper filter and a heavy wire screen which differs from our heavy screen in that it is more loosely woven. The mixture was covered with a cotton filter pad and a wire screen, over which was placed the wire spring.
The use of this mask ensures that all the air breathed must enter the lungs through the canister. This air passage is entirely independent of leaks in the facepiece, due either to a poor fit about the face or to actual leakage (from a cut or tear) of the fabric itself. The facepiece is readily cleared of poison gases which may leak in. This is accomplished by taking a full inspiration, releasing the noseclip, and exhaling through the nose, which forces the air out around the edges of the facepiece.
On the other hand, this type of mask possesses a number of very obvious disadvantages, particularly from a military point of view:
The extreme discomfort of the facepiece. This discomfort arises from a number of causes certain of which are inherent in this type of mask, [Pg 200] among them being: (a) the noseclip, (b) the mouthpiece, and (c) the lack of ventilation within the facepiece chamber.
Aside from the actual physical discomfort of the noseclip and mouthpiece, which becomes intense after long periods of wearing, this combination forces upon the wearer an unnatural method of respiration to which it is not only difficult to become accustomed, but which also causes extreme dryness of the throat. The mouthpiece greatly increases salivation and as swallowing is rather more difficult with the nose closed, this adds another extremely objectionable feature.
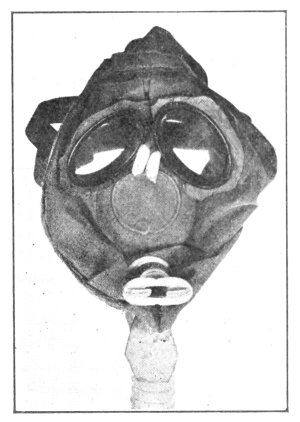
Fig. 45.—Interior of S. B. R.,
Showing
Cotton Wrapped Noseclips.

Fig. 46.—French M-2 Mask.
The lack of ventilation in the facepiece chamber entraps the heat radiating from the face and retains the moisture which is constantly evaporating from the skin. This moisture condenses on the eyepieces, and even if cleared away by the use of a so-called anti-dimming paste, usually makes vision nearly impossible. [Pg 201]
M-2 Mask. The early protection of the French Army was obtained from a mask of the type M-2 (Fig. 46).
This mask consists of a number of layers of muslin impregnated with various absorbent chemicals. A typical mask was made up of 20 layers of cheesecloth impregnated with Greasene and 20 layers impregnated with Complexene. These solutions were made up as follows:
| Complexene: | 39.0 lbs. | Hexamethylenetetramine |
| 37.5 lbs. | Glycerine | |
| 27.5 lbs. | Nickel sulfate (NiSO₄·7 H₂O) | |
| 11.8 lbs. | Sodium carbonate (Na₂CO₃) | |
| Water | ||
| Greasene: | 107.0 lbs. | Castor oil |
| 81.0 lbs. | Alcohol (95%) | |
| 10.7 lbs. | Glycerine (90%) | |
| 3.1 lbs. | Sodium hydroxide (NaOH) | |
This mask fits the face tightly and as a consequence the inhaled air can be obtained only by drawing it through the pores of the impregnated fabric. There is no outlet valve. The exhaled air makes its escape through the fabric. The eyepieces are made of a special non-dimming celluloid. The mask is protected from rain by a flap of weather proof fabric, which also protects the absorbent chemicals from deterioration.
At the beginning of the war the United States experimented considerably with the French mask. Several modifications of the impregnating solutions were suggested, as well as methods of application. One of these was to separate the components of the complexene solution and impregnate two separate layers of cloth; this would make a three-layer mask. In view of the phosgene which was in use at that time, the following arrangement was suggested:
This arrangement was more effective than the original French mask and [Pg 202] offered the following protection when tested against the following gases (concentration 1 to 1,000, rate 30 liters per minute):
| Phosgene | 65 minutes |
| Hydrocyanic acid | 60 minutes |
| Chlorine | 60 minutes |

Fig. 47.—Interior View
of M-2 Mask.

Fig. 48.—French Artillery Mask,
Tissot Type.
Tissot Mask. The French deserve great credit for their development of the Tissot type mask. This was first issued to artillerymen, stretcher bearers, and certain other special classes of soldiers to furnish them with protection and yet enable them to work with greater efficiency because of the decrease in resistance to breathing. The mask (Fig. 48) resembles the British box respirator in that it consists of a canister and rubber facepiece, but differs in that the mouthpiece and noseclip are lacking. The inhaled air enters the mask from two tubes which open directly under the eyepieces and [Pg 203] allow the air to sweep across them. This removes, by evaporation, the condensed moisture of the breath from the eyepieces, which otherwise would obstruct the vision. The circulation of the fresh air in the mask also removes and dilutes lachrymatory gases which may filter through the mask. The exhaled air escapes through a simple outlet valve. This type of mask is advantageous because:
This mask, however, was made of thin rubber of great flexibility which, while affording a perfect fit, did not possess sufficient durability to recommend it as the sole defense of the wearer.
The canister is markedly different from all other canisters described in this chapter in that a highly hygroscopic chemical absorbent is used. An approximate determination showed about 70 per cent sodium hydroxide. The use of caustic soda in the canister is made possible by the intermixing of steel wool with the granules of caustic. A layer of absorbent having the appearance of vegetable charcoal is placed at the top of the canister.
The canister has the shape of a rectangular prism 8 × 6½ × 2½ inches; and, owing to the use of steel wool, is large in proportion to the weight of absorbent contained. Valves are supplied which prevent exhalation through the canister. When not in use the opening in the bottom of the canister is plugged with a rubber stopper to protect the absorbents from moisture. The canister is carried against the body and is connected to the facepiece with a flexible rubber-fabric tube.
A. R. S. Mask (Appareil Respiratoire Special). One of the latest types of French mask is the so-called A. R. S. mask, which is based [Pg 204] upon, or at least resembles closely, the German mask. This is a frame mask made from well rubberized balloon material, provided on the inside with a lining of oiled or waxed linen and fitted with a drum which is screwed on. The mask is provided with eyepieces of cellophane, fastened by metal rings into rubber goggles, which are sewed in the mask. A metal mouth-ring is tied in the mask with tape. This ring is placed somewhat higher than in the German mask, in this way reducing the harmful space under the mask. An inlet and outlet valve is placed in the mouth-ring; the first is of mica while the other, which is in direct communication with the interior of the mask, is of rubber. On the inside of the mask, in front of the valves, a baffle is sewed in, whereby the inhaled air is forced to pass in front of the eyepieces to prevent dimming and, at the same time, condensed vapor is prevented from entering the valves.

Fig. 49.—French A. R. S. Mask.
[Pg 205] The mask or head straps are arranged in the same way as on the latest M-2 mask, i.e., one elastic band is placed across the top of the head and the other across the back; the two are joined by an elastic. Below these two straps is an adjustable elastic neck band. The drum is made of metal similar in shape to the German drum and fits in the mouth-ring by means of a thread. It is made tight by a rubber ring as in the German mask. The thread differs from that on the German mask, making an interchange of canisters impossible. The canister or drum includes a bottom screen, springs and wire screens between the layers. It is closed by a perforated bottom. There are three layers. On the top is a thin layer of absorbent cotton. Beneath this is a central layer of charcoal, which is a little finer than the German charcoal. The lower layer consists of soda-lime, mixed with charcoal and zinc oxide and moistened with glycerine.
The early type of German mask probably served as the model for the French A. R. S. mask. The facepiece was made of rubber, which was later replaced by leather because of the shortage of rubber. The following is a good description of a typical German facepiece:
“The facepiece of the German mask was made of one piece of leather, with seams at the chin and at the temples, giving it roughly the shape of the face. The leather was treated with oil to make it soft and pliable, also to render it impervious to gases. The dressed surface was toward the inside of the mask. A circular steel plate, 3 inches in diameter, was set into the facepiece just opposite the wearer’s nose and mouth, with a threaded socket into which the drum containing the absorbents screwed. A rubber gasket (synthetic?) held in place by a sort of pitch cement, secured a gas-tight joint between the drum and the facepiece. There were no valves, both inhaled and exhaled air passing through the canister. The eyepieces were inserted by means of metal rims with leather washers, and were in two parts: (a) a permanent exterior sheet of transparent material (‘cellon’) resembling celluloid, and (b) an inner removable disc which functioned as an [Pg 206] anti-dimming device. This latter appeared to be of ‘cellon’ coated on the side toward the eye with gelatin, and was held in position by a ‘wheel’ stamped from thin sheet metal, which screwed into the metal rim of the eyepiece from the inside. The gelatin prevented dimming by absorbing the moisture, but wrinkled and blistered and became opaque after a few hours’ use, and could not be changed without removing the mask. The edge of the facepiece all around was provided with a bearing surface consisting of a welt of finely woven cloth about one inch wide sewed to the leather. In some instances this welt was of leather of an inferior grade. The edge of the facepiece was smoothed over by a coat of flexible transparent gum, probably a synthetic compound.”

Fig. 50.—German Respirator.
[Pg 207]

Fig. 51.—The German Respirator
[Pg 208] German Canister. The general appearance of the canister (Sept., 1916 Type) is that of a short thick cylinder slightly tapered and having at the smaller end a threaded protrusion or neck by which it is screwed onto the facepiece. The cylinder is about 10 cm. in diameter and about 5 cm. in length. In the canister are three layers of absorbents of unequal thickness separated by disks of fine mesh metal screen. The canister is shipped in a light sheet iron can 10 cm. in diameter and 8 cm. high. The can is shellacked and is lined with paper packing board. The container is made air-tight by sealing with a strip of adhesive tape.
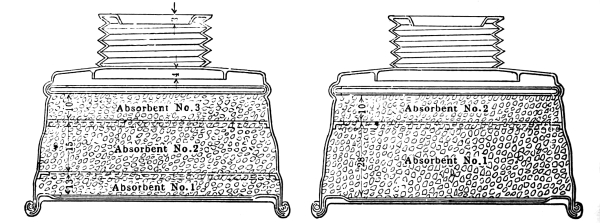
Fig. 52.—Cross Section of 1917 and 1918 German Canisters.
Absorbents.
| Absorbent. | Composition. | Weight. | Volume. | |
|---|---|---|---|---|
| 1917. | No. 1. | Chemical Absorbent. | 66 gr. | 105 cc. |
| No. 2. | Impregnated Charcoal. | 36 gr. | 85 cc. | |
| No. 3. | Chemical Absorbent. | 15 gr. | 45 cc. | |
| 1918. | No. 1. | Impregnated Charcoal. | 58 gr. | 185 cc. |
| No. 2. | Chemical Absorbent. | 29 gr. | 45 cc. | |
| Total Volume of Absorbents, | 1917, 235 cc. = | 14.3 cu. in. |
| 1918, 230 cc. = | 14.0 cu. in. | |
| Total Weight of Absorbents, | 1917, 117 gr. | |
| 1918, 87 gr. | ||
| Volume of Air Space above Absorbents = 50 cc. = 3.1 cu. in. | ||
Body. The body of the canister is made of sheet metal (probably iron), which is protected on the outside with a coat of dark gray paint and on the inside with a japan varnish. For ease in assembling the sides of the canister have a gentle taper, and are formed so as to supply a seat for each of the follower rings. The protrusion or neck has about six threads to the inch, the pitch of the screw being 4 mm. [Pg 209] The lower part of the body is rolled so as to give a finished edge, and the upper part of the cylinder is grooved to receive the top support.
The first screen is double, consisting of a coarse top screen five to six mesh, per linear inch, and immediately below, a finer screen of 30-40 mesh, per linear inch. The top support is a rigid ring of metal with two cross arms, which give added, strength to the ring and support to the screens. It springs into a groove at the top of the body and forms the support for the contents of the canister. Both screens are made of iron wire and the top support is made of iron (probably lightly tinned).
The second screen, which separates the second and third absorbents, is double, consisting of two disks of 30-40 mesh iron screen. Both screens are held in place by a follower ring.
The third screen is single, but otherwise it is exactly similar to the second screen. It serves to keep separate the layers of absorbents No. 1 and No. 2.
The fourth screen (30-40 mesh) is made of iron wire and is held to the bottom support by six cleats which are punched from the body of the support. The bottom support is simply a flanged iron cover for the bottom of the canister. It is punched with 79 circular holes each 4 mm. in diameter and is painted on the outside to match the body of the canister. The screen and the inside of the bottom support or cover are coated with a red paint.
At the entrance of the United States into the war, three types of masks were available: the PH helmet, the British S. B. R. and the French M-2 masks. Experiments were made on all three of these types, and it was soon found that the S. B. R. offered the greatest possibilities, both as regards immediate protection and future development. During the eighteen months which were devoted to improvement of the American mask, the facepiece underwent a gradual evolution and the canister passed through types A to L, with many special modifications for experimental purposes. The latest development consisted in an adaptation [Pg 210] of the fighting mask to industrial purposes. For this reason a rather detailed description of the construction of the facepiece and of the canister of the respirator in use at the close of the war (R. F. K. type) may not be out of place. The mask now adopted as standard for the U. S. Army and Navy is known as the Model 1919 American mask, with 1920 model carrier, and will be described on page 225.
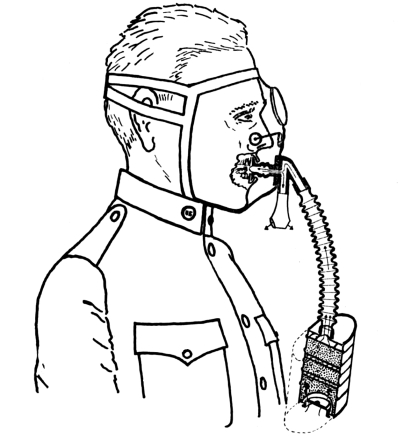
Fig. 53.—Diagrammatic Sketch
of
Box Respirator Type Mask.
Facepiece. The facepiece of the R. F. K. type Box Respirator is made from a light weight cotton fabric coated with pure gum rubber, the finished fabric having a total thickness of approximately ¹/₁₆ inch. The fit of the facepiece is along two lines—first, across the forehead, approximately from temple to temple; second, from the same temporal points down the sides of the face just in front of the ears and under the chin as far back as does not interfere with the Adam’s apple. In securing this fit, the piece of stock for the facepiece is died out of the felt and pleated up around the edges to conform to this line. After this pleating operation, the edges of the fabric are stitched to a binding frame similar to a hat-band made up of felt or [Pg 211] velveteen covered with rubberized fabric. All the stitching and joints in the facepiece are rendered gas-tight by cementing with rubber cement. This facepiece is made in five sizes ranging from No. 1 to No. 5, with a large majority of faces fitted by the three intermediate sizes, 2, 3, 4.
Harness. The function of the harness is to hold the mask on the face in such a way as to insure a gas-tight fit at all points. Because of the great variations in the conformation of different heads, this problem is not a simple one. Probably, the simplest type of harness, as well as the one which is theoretically correct, consists of a harness in which the line of fit across the forehead is extended into an elastic band passing around the back of the head, while the line of fit around the side of the face and chin is similarly extended into another elastic tape passing over the top of the head; these should be held in place by a third tape, preferably non-elastic, attached to the mask at the middle of the forehead and to the middle points of the other tapes at a suitable distance to hold them in their proper positions.
The discomfort of the earlier types of harness has been remedied, in a large measure, by the development of a specially woven elastic web which, for a given change in tension, allowed more than double the stretch of the commercial weaves. There is still much room for valuable work in developing a harness which will combine greater comfort and safety. The following points should always be observed in harness design:
(1) The straps should pull in such a direction that as large a component as possible of the tension of the strap should be available in actually holding the mask against the face.
(2) The number of straps should be kept to a minimum in order to avoid tangling and improper positioning when put on in a hurry by an inexperienced wearer.
Eyepieces. One of the most important parts of the gas mask, from the military point of view, is the eyepiece. The primary requirement of a good eyepiece is that it shall provide a minimum reduction in clarity of vision with a maximum degree of safety to the wearer. The clarity of vision may be affected in one of several ways: (1) by abrasion of the eyepieces under service conditions; (2) irregularities in the surface [Pg 212] and thickness of the eyepiece, causing optical dispersion; (3) absorption of light by the eyepiece itself; (4) dimming of the eyepieces due to condensation of moisture radiating from the face or in the exhaled air.
Three types of eyepieces were used but by the end of the war the first two types had been abandoned.
(1) Ordinary celluloid.
(2) Various hygroscopic forms of celluloid, known as non-dimming eyepieces.
(3) Various combinations of glass and celluloid, known as non-breakable eyepieces.
Celluloid was used first, due to its freedom from breakage. It is not satisfactory because it is rapidly abraded in use, turns yellow, thus increasing its light absorption, has relatively uneven optical surfaces and becomes brittle after service.
The various forms of non-dimming lenses function by absorbing the water which condenses on their surfaces, either by combining individual drops into a film which does not seriously impair vision, by transmitting it through the surface and giving it off on the exterior or by a combination of these mechanisms. With the exception that they are non-dimming, they are open to all the objections of the celluloid eyepiece and, as a matter of fact, were never tried out in the field.
The so-called non-breakable eyepieces are formed by cementing together a layer of celluloid between two layers of glass.[25] This results in an almost perfect eyepiece. Any ordinary blow falling upon such an eyepiece does no more than crack the glass, which remains attached to the celluloid coating. Except in extreme cases, the celluloid remains unbroken and there is relatively slight danger of a cracked eyepiece of this sort leaking gas.
In the matter of flying fragments, the type of eyepiece consisting of a single layer of celluloid and glass with the celluloid placed next to the eye, has probably a slight advantage over the type in which there is glass on both sides. However, the superior optical surface of the latter type, coupled with its greater freedom from abrasion of the surface led to the adoption of this type known as “triplexin” in the mask produced in the later part of the American manufacturing program. [Pg 213] It should be pointed out in connection with this type of eyepiece that it is possible to make it as perfect optically as desired by using the better grades of glass. While the optical properties of these eyepieces undoubtedly suffer somewhat with age, due to the discoloration of the celluloid, it can be safely said that this material, located as it is between the layers of glass and relatively little exposed to atmospheric conditions, will probably be far less affected in this way than is the ordinary celluloid eyepiece.
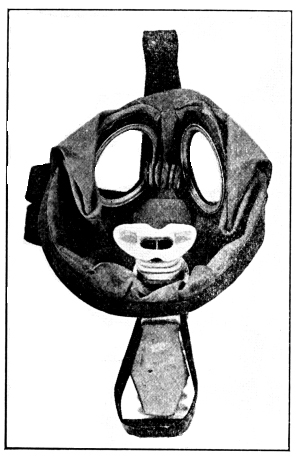
Fig. 54.—American Box
Respirator,
Showing Improved Rubber Noseclip.
The position of the eyepiece is very important; the total and the binocular fields of vision should be kept at a maximum.
Nose clip. The noseclip is probably the most uncomfortable feature of the types of mask used during the War. While a really comfortable nose pad is probably impossible, the comfort of the clip was greatly improved by using pads of soft rubber and springs giving the minimum tension necessary to close the nostrils. [Pg 214]
Mouthpiece. The design of the mouthpiece should consider the size and shape of the flange which goes between the lips and teeth; this should be such as to prevent leakage of gas into the mouth and should reduce to a minimum any chafing of the gums. The opening through the mouthpiece is held distended at its inner end by a metallic bushing to prevent its collapse, if, under stress of excitement, the jaws are forced over the flange and closed. Rubber has proved a very satisfactory material for this part of the facepiece.
Flexible Hose. The flexible hose leads from the angle tube to the canister. This should combine flexibility, freedom from collapse, and extreme physical ruggedness. These specifications are met successfully by the stockinette-covered corrugated rubber hose. The angular corrugations not only give a high degree of flexibility but are extremely effective in preventing collapse. The flexibility gained by this construction is not only lateral but also longitudinal; a hose having a nominal length of 10 inches functions successfully between lengths of 8 and 12 inches. The covering of stockinette, which is vulcanized to the rubber in the manufacturing process, adds materially to the mechanical strength by preventing incipient tears and breaks.
Exhalation Valve. The exhalation valve allows the exhaled air to pass directly to the outside atmosphere. (This valve is not found on the German mask.) This valve has the following advantages:
(1) It tends to reduce very materially the dead air space in the mask.
(2) It prevents deterioration of the absorbent on account of moisture and carbon dioxide of the expired air.
(3) It reduces the back pressure against expiration, since it is unnecessary to breathe out against the resistance of the canister.
The disadvantage, which may under certain conditions be very serious, is that, if for any reason the valve fails to function properly, inspiration will take place through the valve. It can be readily seen that any failure of this nature will allow the poisonous atmosphere to be drawn directly into the lungs of the wearer. [Pg 215]
The type of valve generally used is shown in Fig. 55, which shows one of these valves mounted and unmounted. While it is rather difficult to give a clear description of its construction, the valve may be considered as a flattened triangular sack of rubber, whose altitude is two or three times the base and from which all three corners have been clipped, each giving openings into the interior of the sack. The opening at the top is slipped over the exhalation passage of the angle tube, and the air passes out through the other two corners. Closure is obtained by the combination of two factors,—first, the difference in atmospheric pressure, and second, the tension due to mounting a section which has been cured in the flat over an elliptical opening.
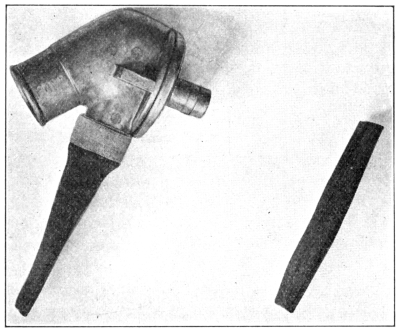
Fig. 55.—American Type
Exhale Valve,
Mounted and Unmounted.
In order to protect the flutter valve from injury and from contact with objects which might interfere with its proper functioning, the later types of valve were provided with a guard of stamped sheet metal.
During the development of the facepiece, as discussed above, the [Pg 216] American canister underwent changes in design which have been designated as A to L. These changes were noted by the different colored paints applied to the exterior of the canister.
Type A canister was exactly like the British model then in use, except that it was made one inch longer because it was realized that the early absorbents were of poor quality. The canister was made of beaded tin plate and was 18 cm. high. The area of the flattened oval section was 65 sq. cm. In the bottom was a fine wire dome 3.4 cm. high. The valve in the bottom was integral with the bottom of the container, there being no removable plug for the insertion of the check valve. The absorbents were held in place by a heavy wire screen on top and by two rectangular springs.
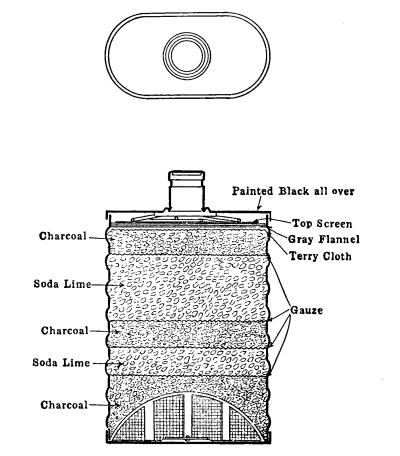
Fig. 56.—American Canister, Type A.
Inhaled air entered through the circular valve at the bottom of the canister, passed through the absorbents and through a small nipple at the top.
The filling consisted of 60 per cent by volume of wood charcoal, developed by the National Carbon Co., and 40 per cent of green soda [Pg 217] lime, developed and manufactured by the General Chemical Company, Easton, Pa. The entire volume amounted to 660 cc. The early experiments with this volume of absorbent showed that ⅖ soda-lime was the minimum amount that could be used and still furnish adequate protection against the then known war gases. It was, therefore, decided to use ⅖ soda-lime and ⅗ charcoal by volume and this proportion has been adhered to in all of the later types of canisters. It is interesting to note that these figures have been fully substantiated by the later experimental work on canister filling.
The charcoal and soda-lime were not mixed but arranged in five layers of equal volume, each layer, therefore, containing 20 per cent of the total volume. The layers were separated by screens of crinoline. At the top was inserted a layer of terry cloth, a layer of gray flannel, and two steel wire screens. The cloth kept the fine particles of chemicals from being drawn into the throat of the person wearing the mask.
This canister furnished very good protection against chlorine and hydrocyanic acid and was fairly efficient against phosgene, but it was useless against chloropicrin. These canisters were never used at the front, but served a very useful purpose as experimental canisters and in training troops.
It was soon found that better protection was obtained if the absorbents were mixed before packing in the canister. This procedure also simplified the method of packing and was used in canister B and following types. Among other changes introduced in later types were: The integral valve was replaced by a removable check valve plug which enabled the men in the field to adjust the valve in case it did not function properly. The mixture of charcoal and soda-lime was divided into three separate layers and these separated by cotton pads. The pads offered protection against stannic chloride smokes but not against smokes of the type of sneezing gas. The green soda-lime was replaced by the pink granules. In April, 1918, the mesh of the absorbent was changed to 8 to 14 in place of 6 to 14.
About July 1, 1918, the authorities were convinced by the field forces of the Chemical Warfare Service that the length of life of the chemical protection of the standard H canister (the type then in use) was excessive and that the resistance was much too high. Type J was [Pg 218] therefore adopted, July 27, 1918. In this the volume of the absorbent was reduced from 450 cc. to 300 cc. It was packed in two layers, ⅔ in the bottom and ⅓ in the top. One pad was placed between the layers and one on top. This change gave a lowering of the resistance of 27 per cent (to 2.5 inches) at a sacrifice of 50 per cent of the length of life of the canister, but not of protection during the shortened life. Type L differed from this only in having 325 cc. of absorbent, a change made to decrease leakage about the top cotton pad.
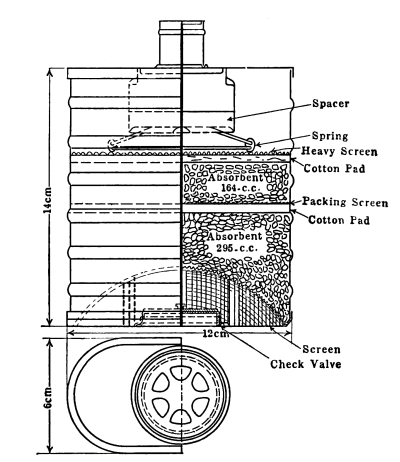
Fig. 57.—U. S. Army Canister, Type J.
The following table shows the relative efficiency of various canisters:
| p. p. m. | U. S., Type H |
British, S. B. R. |
French, A. R. S. |
German | |
|---|---|---|---|---|---|
| Chloropicrin | 1000 | 770 | 17 | 2 | 43 |
| Phosgene | 2500 | 85 | 54 | 5 | 16 |
| Hydrocyanic acid | 500 | 70 | 90 | — | 10 |
| Mustard gas | 100 | 1800 | — | 35 | 195 |
The figures represent time in minutes till the first traces of gas begin to come through. [Pg 219]
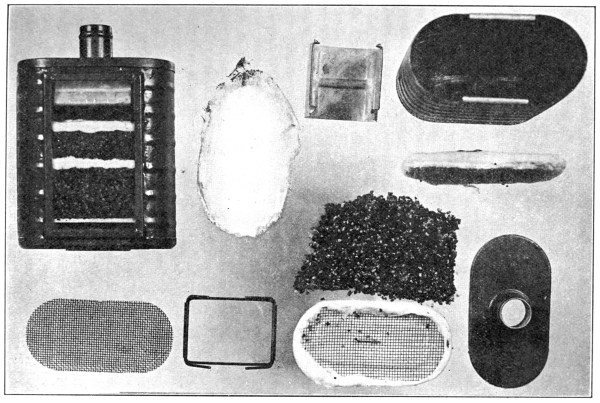
Fig. 58.—Type J Canister and Contents.
[Pg 220]
The following description of the manufacture of the gas mask at the Long Island plant is taken from an article by Col. Bradley Dewey[26]:
“Incoming Inspection—A thorough 100 per cent inspection was made of each part before sending it to the Assembly Department. The inspectors were carefully chosen and were sent to a school for training before they were assigned to this important work. Every feature found to be essential to the manufacture of a perfect gas mask was carefully checked.
“The incoming inspection of the flexible rubber hose leading from the canister to the facepiece can be taken as an illustration. Each piece of hose was given a visual inspection for buckles or blisters in the ends or in the corrugations; for cuts, air pockets, or other defects on the interior; for loose seams where fabric covering was cemented to the rubber tube; for weaving defects in the fabric itself; and for careless application of the cement. Special tests were conducted for flexibility, as a stiff hose would produce a strain on the soldier’s mouth; for permanent set to insure that the hose was properly cured; for the adhesion of the fabric covering to the hose; and for kinking when the hose was doubled on the fingers. Finally each piece was subjected to a test for leaks under water with a pressure of 5 lbs. per sq in.
“Each eyepiece and the three-way metal connection to the facepiece were subjected to a vacuum test for leakage. The delicate exhalation valve was carefully examined for defects which would be liable to cause leakage. Fabric for the facepiece was given a high-tension electrical test on a special machine developed at the plant to overcome the difficulty met in the inspection of this most important material. It was of course necessary that the facepiece fabric be free from defects but just what constituted a defect was the source of much discussion. The electrical test eliminated all personal views and gave an impartial test of the fabric. The machine consisted of two steel rolls between which a potential difference of 4,000 volts was maintained; the fabric [Pg 221] was led through the rolls and wherever there was a pinhole or flaw the current arced through and burned a clearly visible hole.
“Preliminary Facepiece Operations—Blanks were died out from the facepiece fabric in hydraulic presses. Each face blank was swabbed to remove bloom and the eye washers were cemented about the eyeholes. The pockets for holding the noseclips were also cemented to the blanks. The bands which formed a gas-tight seal of the mask about the face were died out from rubberized fabric to which a felt backing was attached. The harness consisting of elastic and cotton tapes was also sewed together at this point.
“Facepiece Operations—The sewing machine operations were next performed. First the died out blanks were pleated to form the facepiece. The operator had to register the various notches in the blank to an accuracy of ¹/₃₂ in. and to locate the stitches in some cases as closely as ¹/₆₄ in. The band was next sewed to the periphery of the facepiece after which the harness was attached. The stitches on the outside of the facepiece were covered with liquid dope, which filled the needle holes and made the seams gas-tight.
“In addition to the inspection of each operation, the completed facepiece was submitted to a control inspection to discover any defects that might escape the attention of the inspectors on the various operations.
“Assembly Operations—The facepieces were now ready for assembly and were sent for insertion of the eyepieces, which was done in specially designed automatic presses. The eyepieces had to be carefully inserted so that the facepiece fabric extended evenly around the entire circumference.
“Before manufacture began on a large scale, the most satisfactory method of conducting each assembly operation was worked out and the details standardized, so that operators could be quickly and efficiently trained. No detail was considered too small if it improved the quality of the mask. The assembly operations proceeded as follows:
“The exhalation valve was first joined to the three-way metal tube which formed the connection between the facepiece, flexible hose, and mouthpiece. Each valve was then tested for leakage under a pressure difference of a one inch head of water. No valve was accepted which showed leakage in excess of 10 cc. per min. under these conditions.
“The metal guard to protect the exhalation valve was next assembled, followed by the flexible hose. The three-way tube was then assembled to the facepiece by means of a threaded connection and the rubber mouthpiece attached. To illustrate the attention to details the following operation may be cited: [Pg 222]
“The contact surfaces between each rubber and metal part were coated with rubber cement before the parts were assembled. The connection was then tightly wired, care being taken that none of the turns of wire should cross and finally the wire was covered with adhesive tape so that no sharp edges would be exposed.
“The masks, completely assembled except for the canisters, were inspected and hung on racks on specially designed trucks which prevented injury in transit, and were delivered to the Finishing Department.
“Canister Filling—Meanwhile the canisters were being filled, in another building.
“The chemicals were first screened in such a way that the fine and coarse materials were separated from the correctly sized materials. They were then carried on a belt conveyor to the storage bins, whence they were fed by gravity through pipes to various mixing machines. A special mixing machine was developed to mix the carbon and granules in the proper proportions for use in the canister. The mixed chemicals were then led to the canister-filling machines. There was a separate mixing machine for each filling machine, of which there were eighteen in all.
“The can-filling department was laid out in six units. Each unit had a capacity of 20,000 cans per day. A system of double belt conveyors was installed to conduct empty canisters to the machines and carry away the filled ones.
“Each filling operation was carefully inspected and special stops were placed on the belt conveyors so that a canister could not go to the next operation without having been inspected. Operators and inspectors were stationed on opposite sides of the belt. The chemicals were placed in the canister in three equal layers which were separated by pads of cotton wadding. The first layer was introduced from the filling machine (which delivered automatically the proper volume of chemicals), the canister was shaken to pack the chemicals tightly, the cotton baffle inserted, the second layer of chemicals introduced and so forth. On top of the top layer of chemicals were placed a wire screen and a specially designed spring which held the contents of the canister securely in place. The metal top was then fitted and securely soldered.
“Each canister was tested under water for possible leaks in joints or soldering, with an air pressure of 5 lbs. per sq. in. A test was also made for the resistance which it offered to breathing, a rate of flow of air through the canister of 85 liters per min. being maintained and the resistance being measured in inches of water. [Pg 223]
“The filled canisters were then painted a distinctive color to indicate the type of filling.
“Finishing Department—In the finishing department, the filled canisters, were conducted down the middle of the finishing tables and assembled to masks.
Fig. 59.
“The finished masks were then inspected, placed in unit boxes, ten to a box, and returned for the final inspection.
“Final Inspection—Final inspection of the completely assembled masks was as rigid as could be devised, and was closely supervised by army representatives. Only the most painstaking, and careful women were selected for this work and the masks were examined in every detail to discover any defect that might have escaped previous [Pg 224] inspection. Finally, each mask was inspected over a bright light in a dark booth for small pinholes which the ordinary visual inspection might not have detected.
“As a check on the quality on the final inspectors’ work a reinspection of 5 per cent of the passed masks was conducted. Where it was found that a particular inspector was making numerous mistakes, her eyes were examined to see whether it was due to faulty eyesight or careless work. Masks containing known defects were purposely sent to these inspectors to determine whether they were capable of continuing the inspection work. In this way the desired standard was maintained.
“A daily report of the final inspection was sent back to each of the assembly departments involved so that defects might be eliminated immediately and the percentage of rejects kept as low as possible.
“After the final inspection the masks were numbered, packed in knapsacks, and the filled knapsacks placed in packing cases, twenty-four to a case.”
The French, as has already been pointed out, early recognized that certain classes of fighting men, as the artillerymen, needed the maximum of protection with the minimum decrease in efficiency. The result of this was the Tissot Mask. Before the United States entered the war, the British standard box respirator had reached a greater degree of perfection, with far greater ruggedness and portability. It was therefore adopted as the American standard. At the time of the invention of the British box respirator and practically up to the time the United States entered the war, masks were worn only during the sporadic gas attacks then occurring and only for a brief period at a time. As the war progressed, the men were compelled to wear their masks for much longer periods (eight hours was not uncommon). It was then seen that more comfort was needed, even at the expense of a little safety.
The principle of the Tissot mask was correct so far as comfort was concerned, since it did away with the irritating mouthpiece and noseclip, but the chief danger in the French mask arose from the fact that the facepiece was made of thin, pure gum rubber. The Research [Pg 225] Division, together with the Gas Defense Division, developed two distinct types of Tissot masks. The first of these was the Akron Tissot, the second the Kops Tissot. The best features of these have been combined in the 1919 Model.
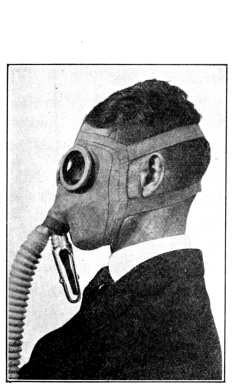
Fig. 60.—American Tissot Mask,
Early Type.
Fig. 61.—American Tissot Mask,
Interior View.
Facepiece. This facepiece is made of rubberized stockinet about one-tenth inch in thickness. The stockinet is on the outside only and is for the purpose of strengthening and protecting the rubber which is of very high grade. The facepiece is died out as a single flat piece from the stockinet which is furnished in long rolls. The die is of such shape that when the facepiece is sewed there is but one seam, and that [Pg 226] between the angle tube opening and the edge under the chin. This seam is sewed with a zigzag stitch with the stockinet sides flat together. The seam is then stretched over a jig, so as to form a flat butt joint. This seam is then cemented with rubber cement and taped, inside and out, to make it thoroughly gas-proof.
The eyepiece openings are of oval shape with the longer axes horizontal and considerably smaller than the finished eyepieces. The eyepieces being circular, the cloth is stretched to accommodate them, giving the necessary bulge to keep the cloth and metal of the eyepieces away from the face. The harness has three straps on each side. Instead of the single strap over the top of the head, two straps lead from directly over the eyes, both being made of elastic the same as the other straps. All six straps are brought together around a pad of felt and cloth about 2½ × 3½ inches at the back of the head. This pad makes the harness much more comfortable.
The rubberized stockinet is reinforced on the inner or rubber side with thin bits of cloth at all points where the straps are sewed on. The strap across the temples just above the ears is sewed at two points, one about one-half inch from the edge and the other about two inches from the edge. This is for the purpose of helping press the cloth against the temples, thereby adding to the gas-tightness for those heads that have a tendency to be hollow at the temples. The lower strap is just above the chin and is for the purpose of giving gas-tightness in that vicinity. All of the straps except the two over the top of the head are attached to the pad with buckles, and are thus capable of exact adjustment.
The eyepieces are of triplex glass in metal rings with rubber gaskets. In pressing the rings home, the rubberized stockinet is turned and held securely so that there is no possibility of pulling them out. The angle tube containing the outlet valve and the connection to the corrugated tube connecting with the canister is the same as with the latest model R. F. K. mask. The only difference as regards the corrugated tube is that a greater length is needed with the new carrier under the left shoulder. The total length of the tube for this model is about 24 [Pg 227] inches. On the inside of the facepiece and connected to the angle tube inlet is a butterfly baffle of rubber, so arranged that the incoming air is thrown upward and over the eyepieces, thus keeping them clear no matter how much the exertion or what the temperature, except in certain rare cases when the temperature is down at zero F. or below.

Fig. 62.—1919 Model American Mask.
[Pg 228]
The canister is radically different from the canisters used in the R. F. K. and earlier types. In the first place, it is longer, the total length finished being 8 inches. It has two inlet valves at the top end protected by a tin cover instead of the single inlet valve at the bottom of the earlier types. The two inlet valves are each ⅝ inch in diameter and are made up of square flat valves on the end of a short rubber tube. The rubber tube is fitted over a short metal tube. Gas-tightness is obtained both by the pressing of the valve against the round edge of the metal tube and by the pressure of the edges against each other. These valves, while delicate, are proving very satisfactory, and being simply check valves to prevent the air going back through the canister, they are not vital. In case of failure, the eyepieces would fog somewhat and the dead air space be increased by that held in the inlet tube.
The canister consists really of two parts—an outer casing that is solid and an inner perforated tin casing. Around the perforated tin is fitted a filter of wool felt ³/₁₆ of an inch in thickness. This wool felt is very securely fastened by turning operations to solid pieces of tin, top and bottom, so that no air can get into the chemicals without passing through the filter. Thus the air coming through the inlet valves at the top circulates around the loosely fitting outside corrugated case to all parts of the filter and after passing through the filter continues through the perforations of the tin into the charcoal and soda-lime granules.
The chemicals are packed around a central wedge-shaped tube extending about two-thirds the length of the can. The wedge is enlarged at the top and made circular where it passes through the top of the can to connect with the corrugated tube. The wedge-shaped inner piece is made of perforated tin and is covered with thin cloth to prevent dust from the chemicals passing into the tube and thus into the lungs. The cans are filled from the bottom and are subjected to two mechanical jarring operations in order to settle the chemicals thoroughly before the [Pg 229] spring which holds them in place is added. The outer tin cap protecting the inlet valves has two openings on each side but none at the ends of the canister.

Fig. 63.—1919 Model American
Mask
after Adjustment.
The carrier is a simple canvas case nearly rectangular, about one foot wide and 15 inches in length. The width is just sufficient at the back to hold the canister and the front part to hold the extra length of corrugated tube and the facepiece. There are two straps, one passing over the right shoulder and the other around the body. The one passing [Pg 230] over the right shoulder has two “V” shaped seams at the top so as to change the direction of the strap over the shoulder in order that it will pull directly downward instead of against the neck. The flap closing the case opens outward.
It has the usual automobile curtain fasteners. A secondary fastener at the top of the opening is arranged so that when the tube is adjusted to the proper length and the mask is adjusted to the face of the wearer, the flap can be buttoned tightly over the corrugated tube and held tightly. This prevents water from entering the case.
Figures 62 and 63 show the position of the carrier both with the facepiece in the carrier and after adjustment. It will be noted that the carrier does not interfere with the pack nor with anything on the front of the body. The left arm hangs almost entirely natural over the case. It has been thoroughly tried out by the Infantry, Cavalry, Artillery and Special Gas Troops and adopted as eminently satisfactory.
Navy. The early Navy canister is a drum much like the German canister. The container is a slightly tapered metal cylinder, 9 cm. in diameter at the bottom. The most satisfactory filling for this drum consists of two layers, 98 cc. in each, of a standard mixture of charcoal and soda-lime, separated by cotton wadding pad. The filling is 6-20 mesh, instead of 8-14 mesh. A later type is shown in Figure 41.
Carbon Monoxide. This canister is discussed in Chapter XI.
Ammonia. Ammonia respirators were needed by the Navy and also by the workmen in refrigeration plants. Early protection was obtained by the use of pumice stone impregnated with sulfuric acid. This had many disadvantages, such as the amount of heat evolved, the caustic fumes produced, high resistance and corrosion of the canister. To overcome these, the “Kupramite” canister was developed. The filling consists of pumice stone impregnated with copper sulfate. Pumice stone, 8 to 14 mesh, and technical copper sulfate are placed in an evaporating pan in the ratio of one part by weight CuSO₄·5H₂O to 1.5 parts pumice, and the [Pg 231] whole is covered with sufficient water to dissolve the salt at boiling temperature. The mixture is then boiled down with constant stirring until crystallization takes place on the pumice and the crystals are nearly dry. The pumice thus treated is then removed from the dish, spread out and allowed to dry in the air. The fines are then screened out on a 14-mesh sieve. Care must be taken in the evaporating process that the absorbent is still slightly moist when taken from the pan.

Fig. 64.—Early Type Navy Mask.
Contains noseclip and mouthpiece.
In packing the standard Army canister with kupramite a layer of toweling is placed on top of the absorbent to filter out any fine particles which might be drawn up from the absorbent, and the whole is held in place by the usual heavy wire screen and spring. This method of packing is to be used with the present mouthpiece type of army mask. If the new Tissot type mask is used, a modification of the packing is desirable in order to eliminate the trouble due to moisture given off by the absorbent during service condensing on the eyepieces of the mask and thus impairing the vision of the wearer. To remedy this defect a [Pg 232] 1-in. layer of kupramite at the top of the canister is replaced by activated charcoal or silica gel, preferably silica gel. This decreases the humidity of the effluent air sufficiently to prevent dimming of the eyepieces. If charcoal is used, a 2-8 cotton pad (Eastern Star Furrier Co., Pawtucket, R. I.) is substituted for the toweling in order to remove charcoal dust. The canister complete weighs about 1.7 lbs.
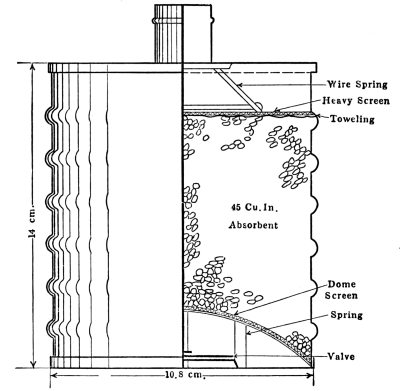
Fig. 65.—Ammonia Canister—“Kupramite.”
A canister containing 45 cu. in. of this material will protect a man breathing at rest for at least 5 hours against 2 per cent ammonia and for 2½ hours against 5 per cent ammonia. Its advantages are large capacity and activity, negligible heat of absorption, and cheapness.
For some time after the introduction of gas warfare, the gases used were of the so-called non-persistent type. Under these conditions it was necessary to wear the mask for only relatively short periods, after [Pg 233] which the cloud dissipated. With the increasing use of gas and the introduction of the more persistent gases, particularly mustard gas, it not only became necessary to wear the mask for long periods of time but also to do relatively heavy physical work, such as serving artillery, when wearing the mask.

Fig. 66.—Ammonia Mask,
Showing Relative Size of Canister.
Under these conditions, it became evident that the wearing of the mask caused a very great reduction in the military efficiency of the soldier. The reasons for this reduction in efficiency have been made the subject of extensive research by a group of the foremost physiologists and psychologists of the country. As a result of their work, the causes contributing to this reduction in efficiency may be grouped about the following main factors:
(1) The physical discomfort of the mask arising from causes such as pressure on the head and face, due to improperly fitting facepieces and harness, the noseclip, and the mouthpiece. [Pg 234]
(2) Abnormal conditions of vision, due to poor optical qualities in eye pieces and restrictions of vision, both as to total field and binocular field.
(3) Abnormal conditions of respiration, among them being (a) the unnatural channels of respiration caused by wearing the box respirator, (b) increase in dead air space in respiratory circuit, and (c) the increase in resistance to both inhalation and exhalation, the last two mentioned being present to a greater or less degree in all types of mask.
Of these general subdivisions the various phases of the first two are so evident that no further discussion will be given. The effects of the changed conditions of respiration are, however, less obvious, and it may be of interest to present in a general way the results of the research along this line, particularly as regards the harmful effects of increasing the resistance and dead air space in the respiratory tract above the normal.
The function of respiration is to supply oxygen to and remove carbon dioxide from the blood as it passes through the lungs. This interchange of gases takes place in the alveoli, a myriad of thin-walled air sacs at the end of the respiratory tract where the air is separated by a very thin membrane through which the gases readily pass. The volume and rate, or in other words, the minute-volume, of respiration is automatically controlled by the nerve centers in such a way that a sufficient amount of air is supplied to the lungs to maintain by means of this interchange a uniform percentage of its various constituents as it leaves the lungs. It will be readily seen therefore, that anything which causes a change in the composition of the air presented to the blood in the alveoli will bring about abnormal conditions of respiration.
Inasmuch as the gaseous interchange between the lungs and the blood takes place only in the terminal air sacs it follows that, at the end of each respiration, the rest of the respiratory tract is filled with air low in oxygen and high in carbon dioxide, which on inspiration is drawn back into the lungs, diluting the fresh air. The volume of these passages holding air which must be re-breathed is known as the anatomical dead air space.
Similarly, when a mask is worn the facepiece chamber and any other [Pg 235] parts of the air passage common to inspiration and expiration become additional dead air space contributing a further dilution of oxygen content and contamination by carbon dioxide of the inspired air in addition to that occasioned by the anatomical dead space, which of course, is always present and is taken care of by the functions normally controlling respiration.
Major R. G. Pearce who directed a large amount of the research along this line, sums up the harmful effects of thus increasing the dead air space as follows:
1. Interpretation from the physiological standpoint:
(a) A larger minute-volume of air is required when breathing through dead air space. This, interpreted on physiological grounds, means that the carbon dioxide content of the arterial blood is higher than normal. The level to which the content of carbon dioxide in the arterial blood may rise is limited. Anything which wastefully increases the carbon dioxide level of the blood decreases the reserve so necessary to a soldier when he is asked to respond to the demand for exercise which is a part of his daily life.
(b) A larger minute-volume of air must be pulled through the canister, which offers resistance proportional to the volume of air passing through it. If resistance is a factor of harm, dead air space increases that harm, since dead air space increases the volume of air passing through the canister.
(c) As will be noted below, the effect of resistance is a tendency to decrease the minute-volume of air breathed. Dead air space increases the minute-volume. Accordingly, if breathing is accomplished against resistance and through a large volume of dead air space, the volume of air breathed is reduced more in proportion to the actual needs of the body than when breathing against resistance without the additional factor of dead space; this, again, causes the level of carbon dioxide in the blood and tissues to be raised to a higher level than normal, and thus again there is some reserve power wasted.
2. Interpretation from the standpoint of the canister.
The life of the canister depends on the volume of the gas-laden air passed through it. The dead space increases the minute-volume of air passed through the canister and, therefore, shortens its life. [Pg 236]
Physiologically, the reason for the harmful effects of breathing resistance is more involved:
“The importance of resistance to breathing lies in: (1) the effect on the circulation of the blood, and (2) the changes in the lung tissue, which seriously interfere with the gas exchange between the outside air and the blood. Data have been presented to draw attention to the seriousness of resistance to inspiration. In these reports, it was suggested that the deleterious effects on the body consist in changes in the blood pressure, increased work of the right side of the heart, and an increase in the blood and lymph content of the lungs. Resistance also decreases the minute-volume of air breathed and thereby increases the percentage of carbon dioxide in the expired air. The foregoing changes are all deleterious.
“Although the chief problem of resistance in gas mask design concerns inspiration, nevertheless resistance to expiration is an important factor. The expired air of the lungs contains carbon dioxide for which means of escape must be provided. The expiratory act is more passive than the inspiratory act, and resistance to expiration is, therefore, more keenly felt than resistance to inspiration. It is then imperative that the exhale valve be so arranged as to allow for the escape of the entire amount of air during the time of expiration with the least possible resistance. The data of the laboratory indicate that seldom, if ever, do expiratory rates rise above a velocity of 150 to 175 per minute. The effect of resistance to exhalation upon the vital organs of the body is not dissimilar to that of inspiration.”
[Pg 237]
The absorbents used in both the British and American gas mask canister, which afforded a degree of protection far superior to that of any other allied or enemy nation except Germany, consisted of a mixture of charcoal and soda-lime, as described in the preceding chapter. In general, a gas mask absorbent must have certain requirements. These are: absorptive activity, absorptive capacity, versatility, mechanical strength, chemical stability, low breathing resistance, ease of manufacture and availability of raw materials.
Absorptive activity, or a very high rate of absorption, is one of the more important properties of a satisfactory absorbent. A normal man when exercising violently breathes about 60 liters of air per minute, and since inhalation occupies but slightly more than half of the breathing cycle, the actual rate at which gas passes through the canister during inhalation is about 100 liters per minute. Calculated on the basis of the regular army canister, this corresponds to an average linear air velocity of about 80 cm. per second. On the average, therefore, a given small portion of the air remains in contact with the gas absorbent for only about 0.1 second. Besides this, the removal of the toxic material must be surprisingly complete. Though the concentration entering the canister may occasionally be as high as one half per cent, even the momentary leakage of 0.001 per cent (ten parts per million) would cause serious discomfort and the prolonged leakage of smaller amounts would have serious results in the case of some gases. The activity of the present gas mask charcoal is shown by the [Pg 238] fact that it will reduce a concentration of 7000 parts per million of chloropicrin to less than 0.5 part per million in less than 0.03 second.
Of equal importance is the absorptive capacity. That is, the absorbent must be able to absorb and hold large amounts of gas per unit weight of absorbent. Its life must be measured in days against ordinary concentrations of gas. It is further necessary that the gas be held firmly and not in any loose combination which might give up minute traces of gas when air is, for long periods of time, breathed in through a canister which has previously been exposed to gas.
The absorbents used must be of a type which can be relied upon to give adequate protection against practically any kind of toxic gas (versatility). The need of this is apparent when the difficulty of having separate canisters for various gases is considered, as well as the difficulty in rapidly and accurately identifying the gases and the possible introduction of new and unknown gases. Fortunately, practically all of the toxic gases are very reactive chemically or have relatively high boiling points and can therefore be absorbed in large amounts by charcoal.
Absorbents must be mechanically strong in order to retain their structure and porosity under conditions of transport and field use. Further, they must not be subject to abrasion for the production of a relatively small amount of fines would tend to plug the canister or to cause channels through which the gas would pass without being absorbed.
Since the canister is filled several months before it is first used in the trenches, and since the canister may be used over a period of months before it is discarded, it is obviously the ultimate activity and capacity (not the initial efficiency) which determines the value of an absorbent. It must therefore have a very considerable degree of chemical stability. By this is meant that the absorbent itself is not subject to chemical deterioration, that it does not react with carbon dioxide, that it does not disintegrate or become deliquescent even after being used and that it has no corrosive action on the metal container.
In a good general absorbent there must be a proper balance between its various essential qualities, and hence the most suitable mixture will probably always be a compromise. [Pg 239]
The fact that charcoal would condense in its pores or adsorb certain gases, holding them firmly, had been known for a long time.[28] In general, it was known that so-called animal charcoal was the best for decolorizing sugar solutions, that wood charcoal was the best for adsorbing gases and that coke had very little adsorbing or decolorizing power. No one knew the reason for these facts and no one could write a specification for charcoal. The ordinary charcoal used in the scientific laboratory was cocoanut charcoal, since Hunter had discovered more than fifty years ago that this was the best charcoal for adsorbing gases.
The first charcoal designed to offer protection against chlorine and phosgene was made by carbonizing red cedar. Since this had little value against chloropicrin, attention was turned to cocoanut shell as the source of raw material. This charcoal fulfilled the above conditions for a satisfactory absorbent better than any other form tested. It must not be supposed, however, that investigation of carbon stopped with these experiments. In the search for the ideal carbon, practically almost every hard vegetable substance known was tested. Next to cocoanut shells, the fruit pits, several common varieties of nuts abundant in the United States, and several tropical nuts (especially cohune nuts), were found to make the best carbon. Pecan nuts, and all woods ranging in hardness from iron wood down to ordinary pine and fir, were found to be in the second class of efficiency. Among other substances tested were almonds, Arabian acorns, grape seeds, Brazil nut husks, balsa, osage orange, Chinese velvet bean, synthetic carbons (from coal, lamp-black, etc.), cocoa bean shell, coffee grounds, flint corn, corn cobs, cotton seed husks, peanut shells and oil shale. While many of these substances might have been used in an emergency, none of them would produce carbon as efficient, volume for volume, as that of the cocoanut shell and other hard nuts. [Pg 240]
Some idea of the scale of charcoal production may be seen from the requirement for cocoanut shells. When we first began to build masks our demands for carboniferous materials ranged from 40 to 50 tons a day of raw material; by the end of the war, we were in need of a supply of 400 tons of cocoanut shells per day. This demand would absorb the entire cocoanut production of tropical America five times over. (The total production of cocoanuts in Central America, the West Indies and the Caribbean Coast of South America amounted to 131,000,000 nuts annually, equal to a supply of 75 tons of shells daily.) It was equal to one-tenth of the total production of the Orient, which amounted to 7,450,200,000 nuts annually. This large demand always made a reserve supply of charcoal material practically impossible. The “Eat More Cocoanut” campaign started by the Gas Defense more than doubled the American consumption of cocoanut in a brief space of time and in October, 1918, with the help of importation of shell, we averaged about 150 tons of shells per day, exclusive of the Orient.
The first heating of cocoanut shells to make charcoal reduces their weight 75 per cent. It was evident, therefore, that we could more economically ship our oriental supply in the form of charcoal produced on the other side of the Pacific Ocean. A charcoal plant was established in the Philippine Islands and agents were sent to all parts of the Oriental countries to purchase enormous supplies of shells. While the work was only gaining momentum when the Armistice was signed, the plant actually shipped 300 tons of cocoanut shell carbon to the United States and had over 1000 tons on hand November 11, 1918.
In the search for other tropical nuts, it was found that the cohune or corozo nut was the best. These nuts are the fruit of the manaca palm tree. They grow in clusters, like bananas or dates, one to four clusters to a tree, each cluster yielding from 60 to 75 pounds of nuts. They grow principally on the west coast of Central America in low, swampy regions from Mexico to Panama but are also found along the Caribbean coast. The chief virtue of the cohune nut from the charcoal point of view was its extreme thickness of shell; this nut is 3 inches or more in length and nearly 2 inches in diameter but the kernel is very small. Four thousand tons per month were being imported at the time [Pg 241] of the Armistice. A disadvantage in the use of cohune nuts was that their husks contained a considerable amount of acid which rotted the jute bags and also caused the heaps of nuts to heat in storage.
A third source of tropical material was in the ivory nuts used in considerable quantities in this country by the makers of buttons. There is a waste of 400-500 tons per month of this material, which was used after screening out the dust. This material is rather expensive, because it is normally used in the manufacture of lactic acid.
Another great branch of activity in securing carbon supplies was concerned with the apricot, peach and cherry pits and walnut shells of the Pacific Coast. A nation-wide campaign on the part of the American Red Cross was started on September 13, 1918. Between this time and the Armistice some 4,000 tons of material were collected. Thus the slogan “Help us to give him the best gas mask” made its appeal to every person in the United States.
It has been pointed out that the first charcoal was made from red cedar. While this was very satisfactory when tested against chlorine, it was of no value against chloropicrin. In order to improve the charcoal still further it was desirable to have some theory as to the way charcoal acted. It was generally agreed that fine pores were essential. The functioning of charcoal depends upon its adsorptive power and this in turn upon its porosity. The greater the ratio of its surface to its mass, that is, the more highly developed and fine grained its porosity, the greater its value. Another factor, however, seemed to play a rôle. As a pure hypothesis, at first, Chaney assumed that an active charcoal could only be secured by removing the hydrocarbon which he assumed to be present after carbonization. Being difficultly volatile, these hydrocarbons prevent the adsorption of other gases or vapors on the active material. To prove this, red cedar charcoal was heated in a bomb connected with a pump which drew air through the bomb. Although the charcoal had been carbonized at 800°, [Pg 242] various gases and vapor began to come off at 300°, and when cooled, condensed to crystalline plates.
This experiment not only proved the existence of components containing hydrogen in the charcoal, but also showed that one way of removing the hydrocarbon film on the active carbon was to treat with an oxidizing agent.
In the light of the later experimental work Chaney feels that there are two forms of elementary carbon—“active” and “inactive”; the active form is characterized by a high specific adsorptive capacity for gas while the inactive form lacks this property. In general the temperature of formation of the active form is below 500-600° C. The form is easily attacked by oxidizing agents—while the latter is relatively stable. The combination of active carbon with an adsorbed layer or layers of hydrocarbon is known as “primary” carbon. Anthracite and bituminous coal are native primary carbons, while coke contains a considerable amount of inactive carbon, resulting from the decomposition of hydrocarbon during its preparation.
“On the basis of the above discussion, the preparation of active charcoal will evidently involve two steps:
“First.—The formation of a porous, amorphous base carbon at a relatively low temperature.
“Second.—The removal of the adsorbed hydrocarbons from the primary carbon, and the increase of its porosity.
“The first step presents no very serious difficulties. It involves, in the case of woods and similar materials, a process of destructive distillation at relatively low temperatures. The deposition of inactive carbon, resulting from the cracking of hydrocarbons at high temperatures, must be avoided. The material is therefore charged into the retorts in thin layers, so that the contact of the hydrocarbon vapors with hot charcoal is avoided as much as possible. Furthermore, most of the hydrocarbon is removed before dangerous temperatures are reached. A slight suction is maintained to prevent outward leaks, but no activation by oxidation is attempted, as this can be carried on under better control and with less loss of material in a separate treatment. [Pg 243]
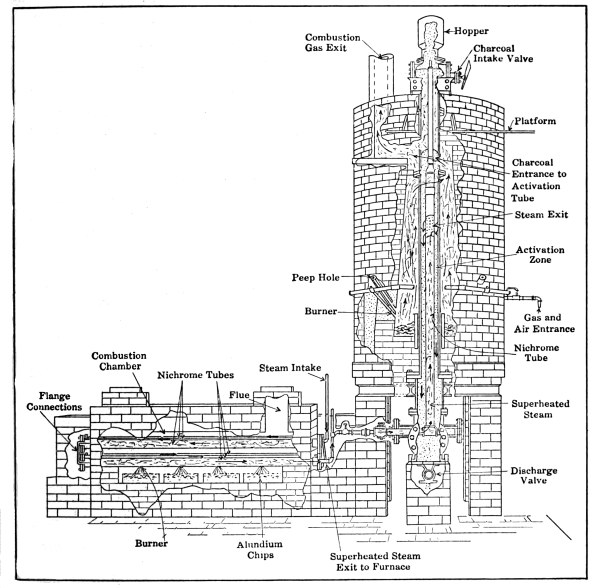
Fig. 67. Dorsey Reactor
for Activating Cocoanut Charcoal with Steam.
“The second step, that is, the removal of the absorbed hydrocarbons from the primary carbon, is a much more difficult matter. Prolonged heating, at sufficiently high temperatures, is required to remove or break up the hydrocarbon residues. On the other hand, volatilization and cracking of the hydrocarbons at high temperatures is certain to produce an inactive form of carbon more or less like graphite in its visible characteristics, which is not only inert and non-adsorbent, but is also highly resistant to oxidation. The general method of procedure which has yielded the best results, is to remove the adsorbed hydrocarbons by various processes of combined oxidation and distillation, whereby the hydrocarbons of high boiling points are broken down into more volatile substances and removed at lower temperatures, or under conditions less likely to result in subsequent deposition of inactive carbon. Thin layers of charcoal and rapid gas currents are used so that contact between the volatilized hydrocarbons and the hot active charcoal may be as brief as possible. In this way [Pg 244] cracking of the hydrocarbons at high temperature, with consequent deposition of inactive carbon, is largely avoided.
“While the removal of the hydrocarbons by oxidation and distillation is the main object of the activation process, another important action goes on at the same time, namely, the oxidation of the primary carbon itself. This oxidation is doubtless advantageous, up to a certain point, for it probably at first enlarges, at the expense of the walls of solid carbon, cavities already present in the charcoal, thus increasing the total surface exposed. Moreover, the outer ends of the capillary pores and fissures must be somewhat enlarged by this action and a readier access thus provided to the inner portions of the charcoal. However, as soon as the eating away of the carbon wall begins to unite cavities, it decreases, rather than increases, the surface of the charcoal, and a consequent drop in volume activity, that is in the service time, of the charcoal, is found to result.
“It is obvious, therefore, that conditions of activation must be so chosen and regulated as to oxidize the hydrocarbons rapidly and the primary carbon slowly. Such a differential oxidation is not easy to secure since the hydrocarbons involved have a very low hydrogen content, and are not much more easily oxidized than the primary carbon itself. Furthermore, most of the hydrocarbons to be removed are shut up in the interior of the granule. On the one hand, a high enough temperature must be maintained to oxidize the hydrocarbons with reasonable speed; on the other hand, too high a temperature must not be employed, else the primary carbon will be unduly consumed. The permissible range is a relatively narrow one, only about 50 to 75°. The location of the optimum activating temperature depends upon the oxidizing agent employed and upon other variables as well; for air, it has been found to lie somewhere between 350 and 450°, and for steam between 800 and 1000°.
“The air activation process has the advantage of operating at a conveniently low temperature. It has the disadvantage, that local heating and an excessive consumption of primary carbon occur, so that a drop in volume activity results from that cause before the hydrocarbons have been completely eliminated. As a consequence, charcoal of the highest activity cannot be obtained by the air activation process.”
The steam activation process has the disadvantage that it operates at so high a temperature that the regulation of temperature becomes difficult and other technical difficulties are introduced. It has the [Pg 245] advantage that local heating is eliminated. The hydrocarbons can, therefore, be largely removed without a disproportionate consumption of primary carbon. This permits the production of a very active charcoal.
It has the further advantage that it worked well with all kinds of charcoal. Inferior material, when treated with steam, gave charcoal nearly as good as the best steam-treated cocoanut charcoal. Because of the shortage of cocoanut, this was a very important consideration.
Fig. 68.—Section of Raw
Cocoanut Shell.
Magnified 146½ diameters.
The air, steam and also carbon dioxide-steam activation processes have all been employed on a large scale by the Chemical Warfare Service for the manufacture of gas mask carbon. [Pg 246]
Fig. 69.—Section of Carbonized Cocoanut
Charcoal.
Magnified 146½ Diameters.

Fig. 70.—Two-Minute Charcoal
not Activated.
Magnified 732 Diameters.
[Pg 247]
“The above considerations are illustrated fairly well by the photo-micrographs shown in Figs. 68 to 71. Fig. 68 shows a section of the original untreated cocoanut shell crosswise to the long axis of the shell. In it can be seen the closely packed, thick-walled so-called ‘stone-cells’ characteristic of all hard and dense nut shells. Fig. 69 is a photograph of a similar section through the same cocoanut shell after it has been carbonized. As these photographs are all taken with vertical illumination against a dark background, the cavities, or voids, and depressions all appear black, while the charcoal itself appears white. It is clear from this photograph that much of the original grosser structure of the shell persists in the carbonized products. Figs. 70 and 71 are more highly magnified photographs of a carbonized charcoal before and after activation, respectively. As before, all the dark areas represent voids of little or no importance in the adsorptive activity of the charcoal, while the white areas represent the charcoal itself. In Fig. 70 (unactivated) the charcoal itself between the voids it seen to be relatively compact, while in Fig. 71 (activated) it is decidedly granular. This granular structure, just visible at this high magnification (1000 diameters), probably represents the grosser porous structure on which the adsorption really depends. These photographs, therefore, show how the porosity is increased by activation.”
Fig. 71.—31-Minute Steam
Activated Charcoal.
Magnified 732 Diameters.
The great demand for charcoal, and the need for activating other than [Pg 248] cocoanut charcoal led to the development of the Dressler tunnel kiln, which seemed to offer many advantages over the Dorsey type of treater.
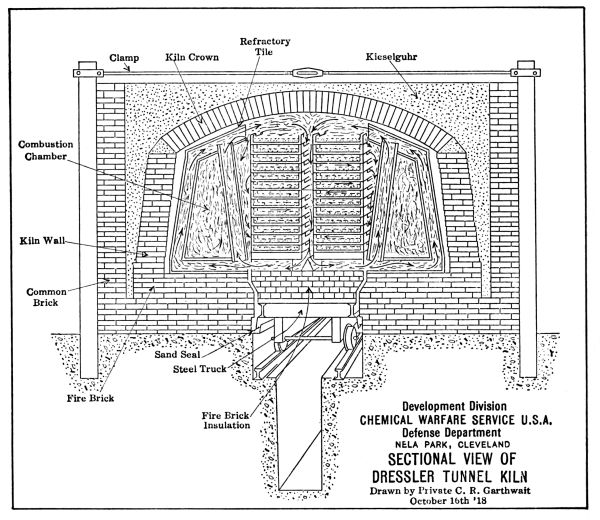
Fig. 72.—Sectional View
of Dressler Tunnel Kiln,
Adapted to Activation of Charcoal.
“The Dressler tunnel kiln is a type used in general ceramic work. The furnace consists essentially of a brick kiln about 190 ft. long, 12 ft. broad, and 9 ft. high, lined with fire brick. Charcoal is loaded in shallow, refractory trays in small tram cars, about 120 trays to the car. The cars enter the kiln through a double door and the charcoal remains in the hot zone at a temperature of about 850° C. for about 4 hrs., depending upon the nature of the material charged. Water is atomized into this kiln, and a positive pressure maintained in order to exclude entrance of air. The kiln is gas-fired and the charcoal is activated by the steam in the presence of the combustion gases. [Pg 249]
“Under such treatment the charcoal is given a high degree of activation without the usual accompanying high losses. Seemingly the oxidizing medium used, together with the operating conditions, produce a deep penetration of the charcoal particles without increasing the extensive surface combustion experienced in the steam activators. The capacity of such a type furnace is limited only by the size of the installation.
“The advantages of this type furnace may be tabulated as follows:
The first experiments were made with a special anthracite coal (non-laminated and having conchoidal fracture). This had a life of 560 minutes as against 360 minutes for air treated cocoanut charcoal and 800-900 minutes for steam-treated charcoal.
When the Gas Defense Service tried to activate anthracite on a large scale in vertical gas retorts at Derby, Connecticut, the attempt was a failure. They carbonized at 900° and then turned on the steam with the result that the steam-treated coal had a slightly greater density than the untreated, which was wrong, and had a shiny appearance in parts with roughened deposits in other parts. When the hydrocarbons are decomposed at high temperatures, the resulting carbon is somewhat graphitic, is itself inactive, is not readily oxidized, and impairs or prevents the activation of the normal carbon upon which it is deposited. This discovery made it possible to treat anthracite successfully. The conditions must be such as to minimize high temperature cracking, to carry off or oxidize the hydrocarbons as fast as formed, and especially to prevent the gases from cooler [Pg 250] portions of the treater coming in contact with carbon at a much higher temperature. With these facts in mind, a plant was built at Springfield which produced 10 tons a day of 150-300 minute charcoal from raw anthracite. This was one-third of the total production at that time and was mixed with the nut charcoal made at Astoria, thereby preventing an absolute shortage of canister-filling material in October, 1918.
It was next shown that the cocoanut charcoal fines resulting from grinding and screening losses and amounting to 50 per cent of the product, could be very finely ground, mixed with a binder, and baked like ordinary carbon products. By avoiding gas-treating in the bake, the resulting charcoal is nearly as good as that from the original shell. A recovery plant for treating the cocoanut fines was built at Astoria. The product was called “Coalite.”
The great advantage of cocoanut shell as a source of charcoal is that it is very dense and consequently it is possible to convert it into a mass having a large number of fine pores, whereas a less dense wood, like cedar, will necessarily give more larger pores, which are of relatively little value. The cocoanut charcoal is also pretty resistant to oxidation which seems to make selective oxidation a more simple matter. By briquetting different woods, it is possible to make charcoal from them which is nearly equal to that from cocoanut shell.
By heating lamp-black with sulfur and briquetting, it was possible to make a charcoal having approximately the same service time as cocoanut charcoal. A charcoal was made by emulsifying carbon black with soft pitch, which gave the equivalent of 400 minutes against chloropicrin before it had been steam-treated. This looked so good that the plans were drawn for making a thousand pounds or more of this product at Washington to give it a thorough test. This was not done on account of the cessation of all research work. The possible advantage of this product was the more uniform distribution of binder.
Instead of steam-treating anthracite coal direct, it was also pulverized, mixed with a binder, and baked into rods which were then [Pg 251] ground and activated with steam. The resulting material, which was known as Carbonite, had somewhat less activity than the lamp-black mixes but was very much cheaper. A plant was built to bake 40 tons a day of this material, which would yield 10 tons a day of active carbon after allowing for grinding losses and steam treatment. The plant was guaranteed to furnish an absorbent having a life of 600 minutes against chloropicrin.
After the Armistice was signed, Chaney took up the question of how the Germans made their charcoal. The German charcoal was made from coniferous wood and was reported to be as good as ours, in spite of the fact that they were using inferior materials. Inside of a month Chaney had found out how the German charcoal was made, had duplicated their material, and had shown that it was nothing like as good as our charcoal. The Germans impregnated the wood with zinc chloride, carbonized at red heat, and washed out most of the zinc chloride. When this zinc chloride was found in the German charcoal, it was assumed that it had been added after the charcoal had been made. It was therefore dissolved out with hydrochloric acid, thereby improving the charcoal against chloropicrin. The German charcoal was then tested as it stood, including the fines, against American charcoal, 8 to 14 mesh. The most serious error, however, was in testing only against a high concentration of chloropicrin. The German charcoal contains relatively coarse pores which condense gases at high concentrations very well but which do not absorb gases strongly at low concentrations. The result was that the German charcoal was rated as being four or five times as good as it really was. [Pg 252]
German Charcoal. ×200.

Fig. 73.—Charcoal from Spruce Wood.
[Pg 253]
The following table shows a comparison of charcoals from different sources. The method of activation was identical and the times of treatment were those approximately giving the highest service time. The results against chloropicrin, therefore, represent roughly the relative excellence of the charcoal obtainable from various raw materials, using this method of activation:
Comparison of Various Active Charcoals Activated in Laboratory
| Base Material | Apparent Density |
Steam Treatment at 900° |
Accelerated Chloropicrin Test Results |
|||
|---|---|---|---|---|---|---|
| Primary Carbon |
Activated Carbon |
Time Min. |
Weight Loss Per Cent |
Weight Absorbed Per Cent |
Service Time Min. |
|
| Sycamore | 0.158 | 0.080 | 18 | 53 | 41 | 7.3 |
| Cedar | 0.223 | 0.097 | 60 | 88 | 78 | 16.0 |
| Mountain mahogany | 0.420 | 0.236 | 60 | 44 | 32 | 16.3 |
| Ironwood | 0.465 | 0.331 | 60 | 44 | 31 | 20.8 |
| Brazil nut | 0.520 | 0.316 | 120 | 71 | 46 | 32.2 |
| Ivory nut | 0.700 | 0.460 | 120 | 70 | 48 | 47.0 |
| Cohune nut | 0.659 | 0.502 | 120 | 48 | 51 | 53.4 |
| Babassu nut | 0.540 | 0.322 | 210 | 68 | 85 | 58.7 |
| Cocoanut | 0.710 | 0.445 | 120 | 60 | 61 | 58.4 |
| Cocoanut | 0.710 | 0.417 | 180 | 75 | 72 | 64.4 |
| Briquetted Materials |
||||||
| Sawdust | 0.542 | 0.365 | 120 | 66 | 53 | 40.0 |
| Carbon black | 0.769 | 0.444 | 240 | 64.3 | 53 | 50.5 |
| Bituminous coal | 0.789 | 0.430 | 165 | 61 | 58.3 | 46.8 |
| Anthracite coal | 0.830 | 0.371 | 480 | 81 | 53 | 40.7 |
“In conclusion, it will be of interest to compare the charcoals manufactured and used by the principal belligerent nations, both with one another and with the above mentioned laboratory preparations. Data on these charcoals are given in the following table: [Pg 254]
Comparison of Typical
Production Charcoals
of the Principal Belligerent Nations
| Country | Date | Raw Material | Apparent Density |
Service Time Corr. to 8-14 Mesh |
Remarks |
|---|---|---|---|---|---|
| U. S. A. | Nov. 1917 | Cocoanut | 0.60 | 10 | Air activated |
| U. S. A. | June, 1918 | Mixed nuts, etc. | 0.58 | 18 | Steam activated |
| U. S. A. | Nov. 1918 | Cocoanut | 0.51 | 34 | Steam activated |
| England | 1917 | Wood | 0.27 | 6 | Long distillation |
| England | Aug. 1918 | Peach stones, etc. |
0.54 | 16 | |
| France | 1917-18 | Wood | 0.23 | 2 | |
| Germany | Early | Wood | ? | 3 | Chemical and steam treatment |
| Germany | June, 1917 | Wood | 0.25 | 33 | Chemical and steam treatment |
| Germany | June, 1918 | Wood | 0.24 | 42 | Chemical and steam treatment |
“It is at once evident that the service time of most of these charcoals is very much less than was obtained with the laboratory samples. However, in the emergency production of this material on a large scale, quantity and speed were far more important than the absolute excellence of the product. It will be noted, for instance, that the cocoanut charcoal manufactured by the United States, even in November, 1918, was still very much inferior to the laboratory samples made from the same raw material. This was not because a very active charcoal could not be produced on a large scale, for even in May, 1918, the possibility of manufacturing a 50-min. charcoal on a large scale had been conclusively demonstrated, but this activation would have required two or three times as much raw material and five times as much apparatus as was then available, due to the much longer time of heating, and the greater losses of carbon occasioned thereby.
“It should furthermore be pointed out that the increase in the chloropicrin service time of charcoal from 18 to 50 min. does not represent anything like a proportionate increase in its value under field service conditions. This is partly due to the fact that the increased absorption on the high concentration tests is in reality due to condensation in the capillaries, which, as has been pointed out, is not of much real value. More important than this, however, is the fact that most of the important gases used in warfare are not held by adsorption only, but by combined adsorption and chemical reaction, for which purpose an 18-min. charcoal is, in general, almost as good as a 50-min. charcoal.”
[Pg 255]
Typical Absorptive Values of
Different Charcoals
Against Various Gases
| No. | Charcoal | Nation | (A) | (B) | Service Time, Minutes Standard Conditions |
||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| (C) | (D) | (E) | (F) | (G) | (H) | (I) | |||||
| 1 | Poor cocoanut | U. S. A. | 0 | 10 | 120 | 175 | 20 | 18 | 55 | 50 | 270 |
| 2 | Medium cocoanut | U. S. A. | 0 | 30 | 350 | 260 | 25 | 25 | 65 | 65 | 370 |
| 3 | Good cocoanut | U. S. A. | 0 | 60 | 620 | 310 | 27 | 30 | 75 | 70 | 420 |
| 4 | Same as No. 2 but wet | U. S. A. | 12 | 18 | 320 | 330 | 35 | 16 | 35 | 95 | |
| 5 | No. 2 impregnated | U. S. A. | 0 | 35 | 400 | 700 | 70 | 400 | 70 | 190 | 510 |
| 6 | Wood | French | 0 | 2.5 | 25 | 75 | 9 | 0 | 1 | 20 | |
| 7 | Wood | British | 0 | 6 | 70 | 90 | 18 | 4 | 5 | 30 | |
| 8 | Peach stone | British | 0 | 16 | 190 | 135 | 30 | 25 | 65 | 60 | |
| 9 | Treated wood | German | 0 | 42 | 230 | 105 | 20 | 20 | 22 | 25 | |
| 10 | No. 9 impregnated | German | 30 | 9 | 90 | 320 | 16 | 1 | 110 | 120 | |
Standard Conditions of Tests
| Mesh of absorbent | 8-14 |
| Depth of absorbent layer | 10 cm. |
| Rate of flow per sq. cm. per min. | 500 cc. |
| Concentration of toxic gas | 0.1 per cent |
| Relative humidity | 50 per cent |
| Temperature | 20° |
| Results expressed in minutes to the 99 per cent efficiency points. | |
| Results corrected to uniform concentrations and size of particles. | |
Charcoal is not a satisfactory all-round absorbent because it has too little capacity for certain highly volatile acid gases, such as [Pg 256] phosgene and hydrocyanic acid, and because oxidizing agents are needed for certain gases. To overcome these deficiencies the use of an alkali oxidizing agent in combination with the charcoal has been found advisable. The material actually used for this purpose has been granules of soda-lime containing sodium permanganate. Its principal function may be said to be to act as a reservoir of large capacity for the permanent fixation of the more volatile acid and oxidizable gases.
The development of a satisfactory soda-lime was a difficult problem. The principal requirements follow: Its activity is not of vital importance, as the charcoal is able to take up gas with extreme rapidity and then later give it off more slowly to the soda-lime. Absorptive capacity is of the greatest importance, since the soda-lime is relied upon to hold in chemical combination a very large amount of toxic gas. Both chemical stability and mechanical strength are difficult to attain. The latter had never been solved until the war made some solution absolutely imperative.
The exact composition of the army soda-lime has undergone considerable modification from time to time as it has been found desirable to change the raw materials or the method of manufacture. A rough average formula which will serve to bring out the interrelation between the different constituents is as follows:
Composition of Wet Mix
| Per Cent | |
| Hydrated lime | 45 |
| Cement | 14 |
| Kieselguhr | 6 |
| Sodium hydroxide | 1 |
| Water | 33 |
| After Drying | |
| Moisture content | 8 |
| After Spraying | |
| Moisture content | 13 (approx.) |
| Sodium permanganate content | 3 (approx.) |
[Pg 257] Within limits, the method of manufacture is more important than the composition or other variables, and has been the subject of a great deal of research work even on apparently minor details. The process finally adopted consists essentially in making a plastic mass of lime, cement, kieselguhr, caustic soda, and water, spreading in slabs on wire-bottomed trays, allowing to set for 2 or 3 days under carefully controlled conditions, drying, grinding, and screening to 8-14 mesh, and finally spraying with a strong solution of sodium permanganate with a specially designed spray nozzle. The spraying process is a recent development, most of the soda-lime having been made by putting the sodium permanganate into the original wet mix. Many difficulties had to be overcome in developing the spraying process, but it eventually gave a better final product, and resulted in a large saving of permanganate which was formerly lost during drying, in fines, etc.
Lime. The hydrated lime furnishes the backbone of the absorptive properties of the soda-lime. It constitutes over 50 per cent of the finished dry granule and is responsible in a chemical sense for practically all the gas absorption.
Cement. Cement furnishes a degree of hardness adequate to withstand service conditions. It interferes somewhat with the absorptive properties of the soda-lime and it is an open question whether the gain in hardness produced by its use is valuable enough to compensate for the decreased absorption which results.
Kieselguhr. The loss in absorptive capacity due to the presence of cement is in part counterbalanced by the simultaneous introduction of a relatively small weight though considerable bulk, of kieselguhr. In some cases, there seems to be a reaction between the lime and the kieselguhr, which results in some increase in hardness.
Sodium Hydroxide. Sodium hydroxide has two primary functions in the soda-lime granule. In the first place, a small amount serves to give the granule considerable more activity. The second function is to [Pg 258] maintain roughly the proper moisture content. This water content (roughly 13-14 per cent after spraying) is very important, in order that the maximum gas absorption may be secured.
Sodium Permanganate. The function of the sodium permanganate is to oxidize certain gases, such as arsine,[30] and to act as an assurance of protection against possible new gases. The purity of the sodium permanganate solution used was found to be one of the most important factors in making stable soda-lime. It was, therefore, necessary to work out special methods for its manufacture. Two such methods were developed, and successfully put into operation.
Careful selection of other material is also necessary, and this phase of the work contributed greatly to the final development of the form of soda-lime.
[Pg 259]
One of the first necessities in the development of absorbents and gas masks was a method of testing them and comparing their deficiencies. While the ultimate test of the value of an absorbent, canister or facepiece is, of course, the actual man test of the complete mask, the time consumed in these tests is so great that more rapid tests were devised for the control of these factors and the man test used as a check of the purely mechanical methods.
Absorbents should be tested for moisture, hardness, uniformity of sample and efficiency against various gases.
Moisture is simply determined by drying for two hours at 150°. The loss in weight is called moisture.
The hardness or resistance to abrasion is determined by shaking a 50-gram sample with steel ball bearings for 30 minutes on a Ro-tap shaking machine. The material is then screened and the hardness number is determined by multiplying the weight of absorbent remaining on the screen by two.
The efficiency of an absorbent against various gases depends upon a variety of factors. Because of this, it is necessary to select standard conditions for the test. These were chosen as follows:
The absorbent under test is filled into a sample tube of specified diameter (2 cm.) to a depth of 10 cm. by the standard method for filling tubes, and a standard concentration (usually 1,000 or 10,000 p.p.m. by volume) of the gas in air of definite (50 per cent) humidity [Pg 260] is passed through the absorbent at a rate of 500 cc. per sq. cm. per min. The concentration of the entering gas is determined by analysis. The length of time is noted from the instant the gas-air mixture is started through the absorbent to the time the gas or some toxic or irritating reaction product of the gas begins to come through the absorbent, as determined by some qualitative test. Quantitative samples of the outflowing gas are then taken at known intervals and from the amount of gas found in the sample the per cent efficiency of the absorbent at the corresponding time is calculated.
| Per cent efficiency = | p.p.m. entering gas - p.p.m. effluent gas | × 100. |
| p.p.m. entering gas |
These efficiencies are plotted against the minutes elapsed from the beginning of the test to the middle of the sampling period corresponding to that efficiency point. A smooth curve is drawn through these points and the efficiency of the absorbent is reported as so many minutes to the 100, 99, 95, 90, 80, etc., per cent efficiency points.
The apparatus used in carrying out this test is shown in Fig. 74. Descriptive details may be found in the article by Fieldner in The Journal of Industrial and Engineering Chemistry for June, 1919. With modifications for high and low boiling materials, the apparatus is adapted to such a variety of gases as chlorine, phosgene, carbon dioxide, sulfur dioxide, hydrocyanic acid, benzyl bromide, chloropicrin, superpalite, etc.
As the quality of the charcoal increased, the so-called standard test required so long a period that an accelerated test was devised. In this the rate was increased to 1,000 cc. per minute, the relative humidity of the gas-air mixture was decreased to zero, and the concentration was about 7,000 p.p.m. The rate is obtained by using a tube with an internal diameter of 1.41 cm. instead of 2.0 cm.
After an absorbent has been developed to a given point, and is considered of sufficient value to be used in a canister, the materials [Pg 261] are assembled as described in Chapter XII. While the final test is the actual use of the canister, machine tests have been devised which give valuable information regarding the value of the absorbent in the canister and the method of filling.
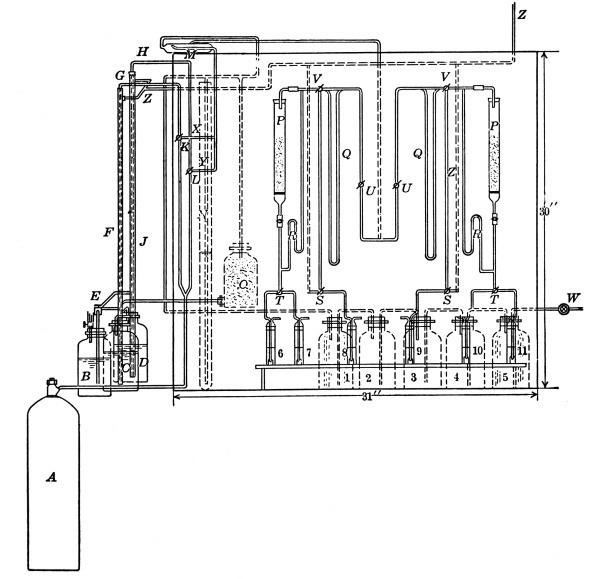
Fig. 74.—Standard
Two-tube Apparatus for Testing Absorbents,
Showing
Arrangement for Gases Stored in Cylinders.
The first test must be that for leakage. The canister must show no signs of leaking when submitted to an air pressure of 15 inches of mercury (about half of the normal atmospheric pressure).
The second factor tested is the resistance to air flow. This is [Pg 262] determined at a flow of 85 liters per minute and should not exceed 3 inches. The latest canister design has a much lower resistance (from 2 to 2½ inches).
The third test is the efficiency of the canister against various gases. For routine work, phosgene, chloropicrin and hydrocyanic acid are used against the standard mixture of charcoal and soda-lime: Chloropicrin is usually used against straight charcoal fillings, while phosgene and hydrocyanic acid are used against soda-lime.
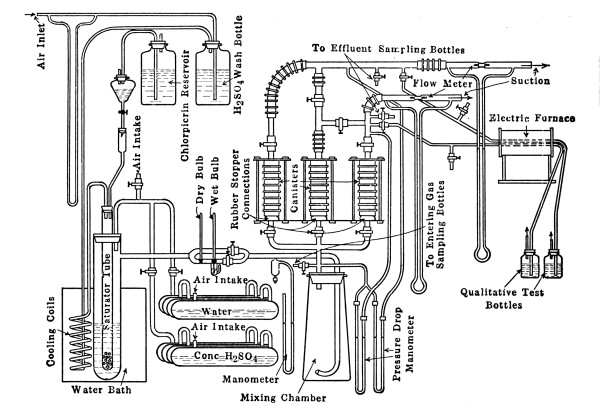
Fig. 75.—Apparatus for Testing Canisters Against Chloropicrin.
Different types of apparatus are required for these gases. They are very complicated, as may be seen from the sketch in Fig. 75, and yet a man very quickly learns the procedure necessary to carry out a test of this kind. The gas is passed through the canister under given conditions, until at the end of the apparatus a test paper or solution indicates that the gas is no longer absorbed but is passing through unchanged. This point is called the “break point,” and the time required to reach this point is known as the life of the canister. This time is also the time to 100 per cent efficiency. Other points, such as [Pg 263] 99, 95, 90 and 80 per cent efficiency are determined. These are used in comparing canisters.
The canister tests were of two general classes: continuous and intermittent. In the first the air-gas mixture was drawn through continuously until the break point was reached. The results obtained in this way, however, did not give the time measure of the value of a canister in actual use. The intermittent test differs only in that the flow of air-gas mixture is intermittent, corresponding to regular breathing. Special valves were adapted to this work.
Canisters must also be tested as to the protection they offer against smoke. These methods are discussed in Chapter XVIII.
The final test of the canister is always carried out by means of the so-called “man test.” Special man-test laboratories were built at Washington, Philadelphia and Long Island. These are so constructed that, if necessary, a man may enter the chamber containing the gas and thus test the efficiency of the completed gas mask. In most cases, however, the canister is placed inside or outside the gas-chamber and the men breathe through the canister, detecting the break point by throat and lung irritation.
The following brief description of the man test laboratory at the American University will give a good idea of the plan and procedure.[32]
The man test laboratory is a one-story building, 56 ft. in length and 25 ft. in width. The main part is occupied by three gas chambers, laboratory tables, and various devices for putting up and controlling gas concentrations in the chambers. A small part at one end is used as an office and storeroom.
Good ventilation is of great importance in a laboratory of this nature. This is secured by means of a 6 ft. fan connected to suitable ducts. The fan is mounted on a heavy framework outside and at one end of the [Pg 264] building. The fan is driven at a speed of about 250 r.p.m. by a 10 h.p. motor. The main duct is 33 in. square, extending to all parts of the building. A connection is also made to a small hood used when making chemical analyses.
The gases, fumes, etc., drawn out by the fan, are forced up and out of a stack 30 in. in diameter, extending upward 55 ft. above the ground level.
The main features of each of the three gas chambers are identical. Auxiliary pieces of apparatus are used with each chamber, the type of apparatus being determined by the characteristics of the gas employed.
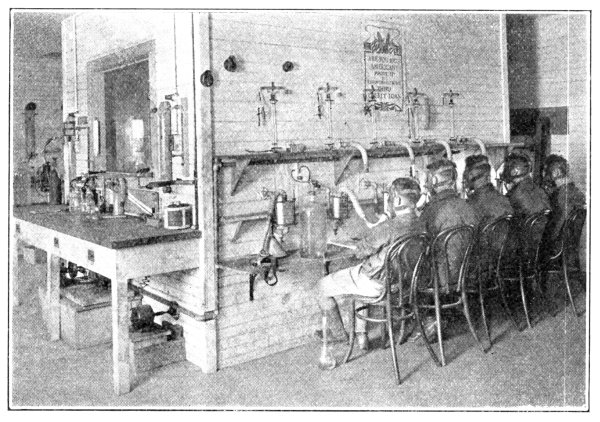
Fig. 76.—Man Test
Laboratory,
American University.
Each chamber is 10 ft. long, 8 ft. wide and 8½ ft. high, having, therefore, a capacity of 680 cu. ft. or 19,257 liters. The floor is concrete, and the walls and ceiling are constructed on a framework of 2 × 4 in. scantling, finished on the outside with wainscoting and on the inside with two layers of Upson board (laid with the joints lapped) covered with a ½ in. layer of special cement plaster laid upon expanded metal lath. The interior finish is completed by two coats of acid-proof [Pg 265] white paint. The single entrance to the chamber is from outside the laboratory, and is closed by two doors, with a 36 × 40 in. lock between them. These doors are solid, of 3-ply construction, 2½ in. thick, with refrigerator handles, which may be operated from either inside or outside the chamber. The door jambs are lined with ³/₁₆ in. heavy rubber tubing to secure a tight seal.
At the end of the chamber opposite the doors, a pane of ¼ in. wire plate glass, 36 × 48 in., is set into the wall, and additional illumination may be secured by 2 headlights, 12 in. square, set into the ceiling of the chamber and of the air-lock, respectively, and provided with 200 watt Mazda lamps and Holophane reflectors. Openings into the chamber, five in number, are spaced across this end beneath the window and 9 in. above the table top.
Fans are installed for keeping the concentration uniform.
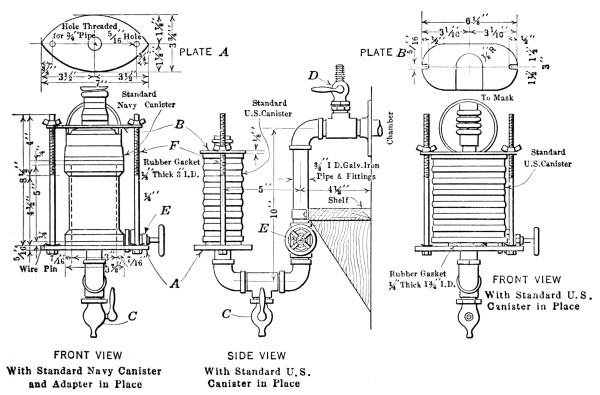
Fig. 77.—Details of Canister Holder.
Various devices have been installed for attaching the canister to be tested (Fig. 77). This arrangement allows the canister to be changed at will without any necessity for disturbing the concentration of gas by entering the chamber. [Pg 266]
Arrangements for removing the gas from the chamber consist of a small “bleeder” which allows a continuous escape of small amounts and a large blower for rapidly exhausting the entire contents of the chamber.
Other general features of the equipment deal with the determination of the physical condition surrounding the tests, often a matter of considerable importance. The temperature of the gas inside the chamber is easily ascertained by means of a thermometer suspended inside the window in such a position as to be read from the outside. The relative humidity of the mixture of air and gas in the chamber is determined by means of a somewhat modified Regnault dew point apparatus mounted on the built-in table.
Another piece of apparatus consists of a combined pressure drop machine and leak tester (Fig. 78) for measuring the resistance of canisters and testing them for faulty construction. This is mounted on a small table, with the motor and air pump installed on a shelf underneath. The resistance, or pressure drop, of canisters is measured by the flow meter A and the water manometer B. Air is drawn through the canister and the flow meter A at the rate of 85 liters per min., the flow being adjusted by the needle valve. The pressure drop across the canister is read on the water manometer B, one end of which is connected to the suction line, the other open to the air. The reading is generally made in inches, correction being made for the resistance of the connecting hose and the apparatus itself.
Canisters are tested for leaks by the apparatus shown at D in Fig. 78. The canister is clamped down tightly by wing nuts against a piece of heavy ¼-in. sheet rubber large enough to cover completely the bottom of the canister and prevent any inflow of air through the valve. Suction is then applied, and a leak is indicated by a steady flow of air bubbles through the liquid in the gas-washing cylinder E. A second gas-washing cylinder, empty, is inserted in the line between E and the canister as a trap for any liquid drawn back when the [Pg 267] suction is shut off. If a leak is shown, it can be located by applying air pressure to the canister and then immersing it in water.
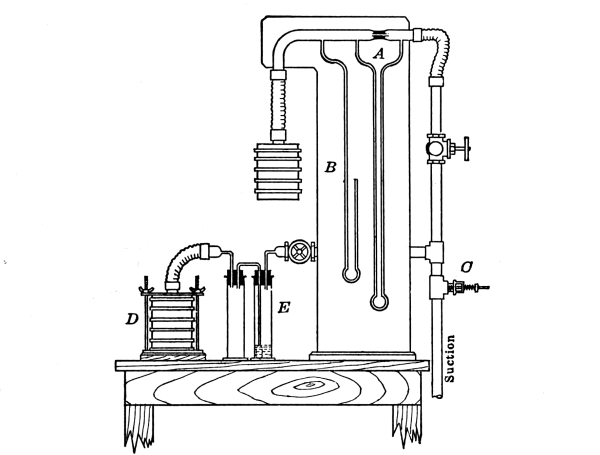
Fig. 78.—Apparatus
for Determining Pressure Drop
and for Detecting Leaks in Canisters.
Three general methods of conducting man tests are followed:
(1) Canisters are placed in the brackets outside the chamber or fastened to the wall tubes within the chamber. The subjects of the test remain outside the chamber, and the facepieces of the masks are connected directly to the canisters, in the first case, and to the wall tubes connecting with the canisters, in the second case. The concentration is established and the time noted. Then the men put on the masks and breathe until they can detect the gas coming through the canisters. Reading matter is provided for the men during the test period. When gas is detected, the time is again noted and the time required for the gas to penetrate the canister is reported as the “time to break down” or “service time” of the canister. Ten canisters are tested at one time, and the average of the results for the 10 canisters is taken for that type of canister. Much less accurate results are [Pg 268] obtained when the final figure is based on a small number of canisters. This is largely due to the various breathing rates and sensitiveness of different men.
(2) The canisters are placed as in (1), but it is only necessary to know if they will give perfect protection for a given length of time. The procedure is the same as in (1), except that the test is arbitrarily stopped at the end of the indicated time, and the number of canisters and the service times of the same noted.
(3) When the canisters are of such a type that they cannot be properly tested as in (1), or when it is desired to test the penetrability of the facepiece, the men wear the complete mask and enter the chamber. They remain until gas penetrates the canister or the facepiece, as the case may be, or until it is determined that the desired degree of protection is afforded. The service time is computed as in (1).
(4) Maximum-breathing-rate tests are made either by men in the chamber or by the men outside, in which they do vigorous work on a bicycle ergometer. In this test the average man will run his breathing rate up to 60 or 70 liters per min.
The concentration of the gas is followed throughout the test by aspirating samples and analyzing them.
Type of Masks Used. In the future the 1919 model will be used for all tests. In general, during the War, the following procedure held, although variations occurred in special cases:
When men entered a gas-chamber, the full facepiece was, of course, required. The type of facepiece was determined by the nature of the gas. If the gas was most easily detected by odor or eye irritation, a modified Tissot mask was used. If it was most easily detected by throat irritation, a mouth-breathing mask was employed.
When men were outside the chamber, the choice was made in the same manner, except in the case of detection of the gas by throat irritation. In this case the mouthpiece was attached to two or three lengths of breathing tubes and a separate noseclip was used. The facepiece was not needed and the men were much more comfortable without it.
Disinfection of Masks. Mouthpieces are disinfected after use by [Pg 269] first holding them under a stream of running water and brushing out thoroughly with a test tube brush; then the latter is dipped into a 2 per cent solution of lysol, and the inner parts of the mouthpiece are brushed out well; finally the mouthpiece and exhaling valve are dipped bodily into the lysol solution and allowed to dry without rinsing. Tissot masks are wiped out with a cloth moistened in alcohol, followed by another cloth moistened in 2 per cent lysol solution. The flexible tubes are given periodic rinsings with 95 per cent alcohol.
Applicability of Man Tests. Man tests are applicable to all gases which can be detected by the subject of the test before he breathes a dangerous amount.
The man test laboratory described above provides facilities for obtaining information concerning the efficiency of canisters, facepieces, etc., within very short periods of time, without waiting for the construction of special apparatus required for machine tests. To get satisfactory results from machine tests, a delicate qualitative chemical test for the gas is essential. Man tests can be made when such a qualitative test is not known. Further, man tests can be made with higher concentrations of some gases than is practicable with machines. Evolution of excessive amounts of moisture when high concentrations of some gases are used causes much more trouble with machine tests than with man tests.
On the other hand, man tests are adversely affected by the varying sensitiveness and lung capacities of the men, and the humidity of the air-gas mixture is not subject to as exact control as is the case with machine tests.
It will be observed that all of the above tests are concerned only with the efficiency of the absorbent and its packing in the canister. No attempt was made to determine the comfort and general “feel” of the mask. For this purpose field tests were devised, covering periods from two to five hours. The first test was a five-hour continuous wearing test. It was assumed that any mask which could be worn for five hours without developing any marked features of discomfort could, if the [Pg 270] occasion demanded it, be worn for a much longer period of time. A typical test follows:
| 8:00 to 8:30 | Instruction and adjustment of gas mask. |
| Gas-chamber tests | |
| 8:30 to 9:30 | Games involving mental and physical activity |
| 9:30 to 11:30 | Cross-country hike with suitable periods of rest |
| 11:30 to 12:00 | Tests of vision |
| 12:00 to 12:30 | Games to test mental condition of subjects |
| 12:30 to 1:00 | Gas-chamber fit test |
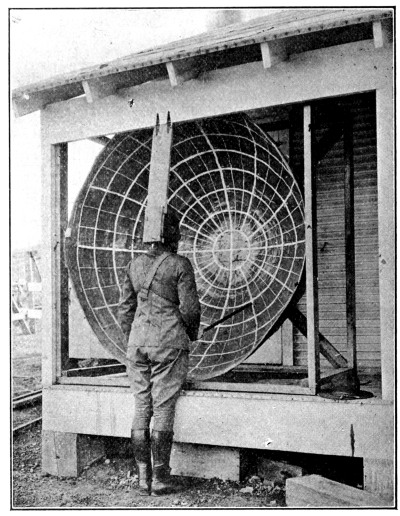
Fig. 79.—Hemispherical Vision Chart.
Vision was tested by means of a hemispherical chart (Fig. 79). This chart was 6 ft. in diameter and was constructed of heavy paper laid over a wire frame. A hinged head rest was provided for holding the [Pg 271] subject’s head firmly in position with the center directly between the eyes. The subject wearing the mask took up his position, and with one eye closed at a time, indicated how far along the meridian of longitude he could see with the other eye. The observer sketched in the limit of vision by outlining the perimeter of the roughly circular field allowed by each eyepiece. The intersection of the two fields gave the extent of binocular vision possible with the mask.
Various other tests were also used, in order that the extent and nature of the vision could be accurately determined.
Aside from the problems of comfort, protection, vision and other important features of gas mask efficiency, the question arose as to whether certain designs of masks or canisters were mechanically able to withstand the rough treatment they were certain to receive in actual field service. A test was, therefore, developed to simulate such service as transportation of masks from base depots to the front, carrying of supplies and munitions by men wearing masks in the “alert” position, exposure to rain and mud, hasty adjustment of masks during gas alarms and typical mistreatment of masks by the soldiers.
All these tests were of great value in the development of a good gas mask.
[Pg 272]
Protective clothing was an additional feature of the general program of protection. As far as factory protection is concerned, the use of protective garments was more or less of a temporary expedient and they were abandoned as fast as automatic machinery and standard practice made their use less necessary. It is likewise a question regarding their value at the front. It is very certain that the garments developed needed to be made lighter and more comfortable to be of much value to the fighting unit.
The first development of protective clothing was along the lines of factory protection. The large number of casualties in connection with the manufacture of mustard gas made it imperative that the workmen be protected not only from splashes of the liquid mustard gas, but also from its vapors. The first suit developed provided protection to the entire body. The ordinary clothing materials and even rubberized fabrics offered little protection but it was found that certain oilcloths were practically impermeable to mustard gas. The suit was a single garment, buttoning in the back, with no openings in the front, no pockets and with tie-strings at wrists and ankles. The head was protected by means of an aluminium helmet, supported by means of a head band resting on the head like a cap and slung from the inside of the helmet; this permitted slight head motions independent of the helmet. In order to provide cooling and ventilating and pure air breathing, the suit was inflated by pumping a considerable volume of air into the suit through a flexible hose long enough to permit considerable freedom of movement. [Pg 273]
This suit had the very great disadvantage of limiting the range of motion to the length of the hose. Because of this, a Tissot type mask was used in place of the helmet and hose connections. The hood was made of the same special oilcloth as the suit, enveloped the head and neck and extended a short distance down the back and over the chest. The canister was slung on the left hip by an oilcloth harness and was kept from swinging by an oilcloth belt around the waist. The canister was much larger than the standard box respirator, had a much longer life with lower resistance and weighed about 3.5 lbs.

Fig. 80.—Impervious Overall
Suit
for Mustard Gas.
Another type of impervious overall suit was developed which protected against mustard gas for over 100 minutes. The material was a cotton sheeting which was impregnated with linseed oil containing a suitable non-drying material, which was thoroughly oxidized in the fabric. These suits proved to be very uncomfortable, especially in warm weather, because they entirely prevented the escape of perspiration from the body. [Pg 274]
Semi-permeable suits were then prepared, in which the cotton sheeting was impregnated or coated with a solution of gelatin and glycerine. The fabric was then “tanned” to render the gelatin insoluble in water. Such a suit is valuable for factory wear, but the impregnating material is easily leached out and the suit is therefore not recommended for field service.
This was built with an inside layer of dry cloth together with an outside layer of treated cloth to afford the necessary chemical protection against mustard gas. Work of fabrication consisted in treating the cloth with simplexene, cutting the suits to design and size, and sewing them together.
Treatment consisted in passing the fabric through a dye machine, then through the wringer rolls where the excess oil was expressed. The inner layer of dry cloth was found necessary, since the cloth was cut as soon as treated. Simplexene does not attain the maximum degree of “tackiness” for two or three days, owing to the presence in the oil of a small amount of volatile spirits. However, by allowing the cloth to air for 48 hours before cutting, the inner lining could probably be dispensed with.
The fighting suits were distributed among various detachments using mustard gas in field tests, and in other places where protection against vapor was needed and where field conditions were approximated. The tests showed that the suit gave satisfactory protection for considerable periods against mustard gas vapors. No other suit, equal both in porosity and protection, has yet been submitted, although samples furnishing better protection with much higher resistance have been examined. The protection of the simplexene suit is about 30 minutes against saturated gas. A large number of these suits were made and taken abroad for field tests at the front.
Protective gloves have been made with a variety of impregnating agents. The one which was selected for large scale production was impregnated with a solution of cellulose nitrate because of the availability of materials and the protection offered by the finished product. The [Pg 275] material is impregnated after being made up. The one finger type of glove is used. The gloves are placed on wooden forms and dipped into the impregnating solution. After draining a few minutes, the gloves are turned upside down on racks and run through a drying oven. Finally they are removed from the forms and conditioned by drying at a moderate temperature for several hours. After being properly cured they are fitted with two straps on the gauntlet of each glove. They should offer protection to chloropicrin (standard method of test) for 30 minutes. When subjected to rough work they will last from one to two weeks.
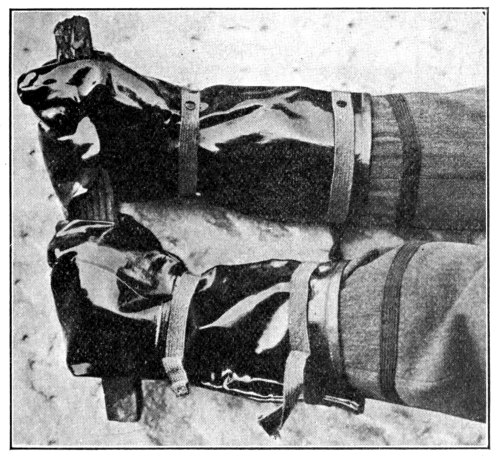
Fig. 81.—Coated Gloves for
Protection against Mustard Gas.
The extensive use of mustard gas on the field caused the men to be exposed to low concentrations of the vapors for extended periods of time. Since it did not seem feasible to furnish the men with special fighting suits, which would protect them against these vapors, it was desirable to provide protection in the form of an ointment which could be applied to the body. In order to be satisfactory an ointment should have the following properties: [Pg 276]
(a) It should protect against saturated mustard gas during the longest possible exposure.
(b) Its protective action should last as long as possible after the application of the ointment. It was felt that the ointment should give protection for 24 hours after it is applied, even if the body is perspiring freely.
(c) The material should not be easily rubbed off under the clothing.
(d) It should be non-irritating to the membranes of the body.
(e) There should be no likelihood of toxic after-effects on long use.
(f) It should be of a good consistency under a fairly wide temperature range and give a good coating at the temperature of the body.
(g) Its method of manufacture should be simple and rapid, and the raw materials required should be abundant.
(h) The cost should not be excessive.
An extensive study of this question was made both in the laboratories and on the field. At first it was believed that successful results could be obtained by the use of such ointments. Careful investigation showed, however, that while these ointments really did protect against rather high concentrations of vapor for short times of exposure, they were probably not so valuable when used against low concentrations over an extended period of time. It was further demonstrated that the protection furnished by a coating of linseed oil is practically equal to the best ointment which has been developed. About 150 ointments were prepared and tested. These consisted of two parts or components, the metallic soap or other solid material and the oil or liquid part which bound and held the solid. The latter is called the base. The best base is lanolin, containing 30 per cent of water. A solution of wax in olive oil was next best. Of the metallic soaps the oleates and linoleates are better than the stearates. A satisfactory ointment has the following composition:
| Zinc oxide | 40 |
| Linseed oil (raw) | 20 |
| Lard | 20 |
| Lanolin | 20 |
[Pg 277] A modification of this formula is:
| Zinc oxide | 45 |
| Linseed oil | 30 |
| Lard | 10 |
| Lanolin | 15 |
The physical properties of this ointment are very good. It forms a smooth, even coating on the skin, sticks well enough not to rub off easily on the clothing and yet is not sticky. Its consistency is such that it can be readily pressed from an ointment tube. A. E. F. reports indicate that sag paste (zinc stearate and vegetable oil) is as satisfactory as any of the preparations tried.
The great difficulties of such preparation from a field point of view are: Extra weight to be carried by the soldiers, necessity for keeping in tight boxes or tubes, thereby adding to the difficulty of carrying, and finally, the difficulty encountered when applying it properly to the body in the field, where gas contaminated hands may cause harm.
The paste was too late a development for thorough field trial. It was used just enough to cause severe partisan controversies between its advocates and those opposed to it. Unquestionably, it proved of decided value in preventing mustard gas burns when properly applied. There are many authentic cases where men alongside each other were similarly gassed except as to burns. The difference in burns arose from the use or non-use of the paste, and in some cases of poor application. Fries is of the opinion that had the war lasted another year the use of pastes would have become universal unless some thoroughly successful substance for impregnating the uniform or underclothing had been developed. This is likewise his belief for the future.
Horse Mask. The need of protection for animals (horses and dogs), although not as great as in the case of men, was of sufficient importance so that masks and boots were developed for the horse and a mask for the dog. [Pg 278]
The German horse mask was the first produced. It was of the nose bag type, enveloping the mouth and nose of the animal. It was fitted with a complicated drawstring and with snap hooks fastening it to the harness. The interior contains a plate of stiff material to prevent the collapse of the bag. The mask itself was apparently not impregnated, but was used wet or with a filling of wet straw or rags to act as the absorbent.
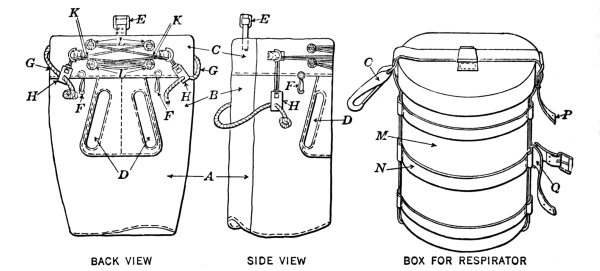
Fig. 82.—German Respirator for Horses.
The French had two types of horse masks impregnated with a glycerine-nickel hydroxide mixture. One type had a closed bottom, while in the other, the bottom was open.
The British horse mask has a two-layer flannelette bag, with a canvas mouth pad and elastic drawstring. It was impregnated with a mixture of phenol, formaldehyde, ammonia, canister soda and glycerine.
The first type of American horse mask was modelled after the British and was impregnated with the Komplexene mixture (hexamethylenetetramine, glycerine, nickel sulfate mixture). This mask had too high a resistance and caused complete exhaustion in running horses. The second mask was made of a large number of layers of very open cheesecloth. It consists of two bags, impregnated with different mixtures (Komplexene and Simplexene). Horses can run two miles with this mask without showing evidences of exhaustion.
Dewey gives the following method of manufacture: [Pg 279]
The chemical employed consisted of a mixture of hexamethylenetetramine (to give protection against phosgene), nickel sulfate (to protect against the possible use of hydrocyanic acid), sodium carbonate and glycerine. This solution was mixed in a heavy steam jacketed mixing kettle with heavy geared stirrers. The mixture was conducted by pipes to the impregnating apparatus which consisted of a rotary laundry washing machine. The masks were treated in this machine for 15 minutes, and then placed in a power operated wringer and the solution driven off to a given weight. Following this operation, they were suspended on wire supports and conducted through a hot air drying machine and dried to a definite weight. 378,000 horse masks were produced at the rate of 5,000 per day.

Fig. 83.—Horse Mask—American Type.
Theoretically, horse masks and horse boots are very valuable,—practically, they did very little actual good in the field, not that they would not protect or that animals would not wear them. The trouble was with the riders and drivers. Gas attacks, coming usually at night, made adjustment of horse masks difficult at best, [Pg 280] while in the confusion of bursting shell and smoke, the drivers absolutely forgot the horse masks or after putting on their own masks feared to try putting masks on the animals. This last was natural as most animals fight the adjustment of the mask and in so doing there is great risk that the man’s mask may be torn off and the man gassed. In the future, such masks will have even more importance than in the past, for the present methods of manufacture of mustard gas coupled with its all-round effectiveness will cause a use of it ten-fold greater than at any time in the World War. In such cases, operations will necessarily be frequently carried on over large areas thoroughly poisoned with mustard gas. Here the animals will be masked and booted before entering the gassed area, and remain so until they leave it. In the torn and broken ground around the front line there will always be need for animal transportation,—wagon, cart and horse—as in such places it is far better in nearly all cases than motor transport.
Dog Mask. The use of dogs in messenger service and in Red Cross work, in which gassed areas must be passed, led to the designing of a mask to give the animals suitable protection. The same materials and method of impregnation were used as in the horse mask. With eight layers of cheesecloth, adequate protection against mustard gas was secured with practically no pressure drop.
The eyepieces were made of thin sheets of cellulose acetate bound around the edge with adhesive tape and sewed directly over openings cut through the mask fabric. The ear pockets were made round and full enough to fit pointed or lop-eared animals. The mask is continued to form a wide neck band which may be drawn up by two adjustable straps. It is made sufficiently full to allow a free movement of the dog’s jaws and yet tight enough around the neck to avoid the possibility of being pawed off. The dog apparently soon became accustomed to wearing the mask.
Horse Boots. The increasing amount of mustard gas used on the Western front made it seem necessary to develop some form of protection for the horse’s hoof and fore-leg. It has been found that mustard gas vapors attack the fleshy portion of the leg, especially around the coronary band and causes inflammation of the frog of the foot. The [Pg 281] problem was solved by devising a special hoof pad and a boot. The pad was made of sheet iron imbedded in a hoof protector (composition rubber) to which the shoe is applied. The shoe just overlaps the metal plate on the inside and provides a solid metal surface for the bottom of the foot. Such a pad not only offers protection against gas but against shell splinters, barbed wire, etc., and would be useful at all times on the front.
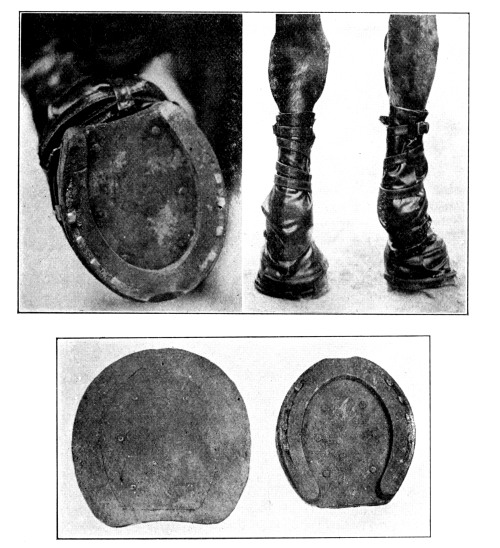
Fig. 84.—Impervious Boots
and Pads to Protect
Horses’ Legs and Hoofs against Mustard Gas.
[Pg 282]

Fig. 85.—Protective Gas
Outfit—Gas Mask, Gas Suit,
Gloves, Boots, Horse Mask, Horse Boots, Horse Pads.
The boot was made of satin, treated so as to be impervious to mustard gas. It covers all of the foot except the bottom and extends to just below the knee. The boot is held in contact with the hoof by a sewed cloth strap, which passes around the bottom of the hoof and is held in position by projections extending from the spur or toe clip. Special care is taken to insure a perfect joint at the rear of the boot since the small cavity in the back of the hoof is one of the most sensitive parts. The boot is wrapped about one and a half times around the leg and is clipped with five loops through which passes a ¾-inch strap. [Pg 283]
Dugout Blankets. Dugout protection is intended to prevent entrance of any gases, lethal, lachrymatory or irritant, into the enclosed space. This has been most efficiently accomplished by means of curtains hung upon wooden frames and fitting closely against all edges of the opening to be closed. These curtains have usually been of heavy material and have generally been spoken of as dugout blankets. Since they were designed to exclude all toxic gases, they had to be devised upon general mechanical principles rather than upon principles of chemical action with specific gases. Permeability to air has not been considered a necessity, it being held that sufficient ventilation is secured by means of the air entering through the soil. For large dugouts and extended use large air filters were designed to draw pure air into the dugout with a fan.
The qualities aimed at, to which both fabric and treatment should contribute, are the following:
Army blankets, both those for men and those for horses, proved suitable materials for curtains, but the scarcity of wool made it desirable to select an all cotton fabric.
A large number of oils were studied as impregnating agents. The most satisfactory mixture consisted of 85 per cent of a heavy steam refined cylinder oil and 15 per cent of linseed oil. This is taken up to the extent of about 300 per cent increase in weight of the blanket during impregnation. It becomes oxidized to some extent upon the surface of the blanket, which becomes less oily than the soft, central core. The finished blanket possessed the following properties: It resists penetration of 400-600 p.p.m. of chloropicrin for 8 hours (dugout test) and mustard gas for 100-400 minutes (machine test). It is sufficiently flexible after standing for 2 hours at 18° F. to unroll of its own [Pg 284] weight, and may be unrolled by applying a slight force at 6° F.; it is not ignited by lighted matches and shows but little loss by drainage.
Two types of machines were designed for impregnation, one for use on large scale behind the line, and a field apparatus for use at the front.
[Pg 285]
The intelligent use of screening smokes in modern infantry tactics offers innumerable advantages through concealment and deception. It confers upon daylight operations many of the advantages which were gained by conducting operations at night with few of the disadvantages of the latter.
Smoke screens have been frequently used by the Navy and by Merchantmen; a common method of escape was to shut off the air from the fire with consequent incomplete combustion of the fuel, thus causing a cloud of dense black smoke. This is often mentioned in the blockade runners of the days in the Civil War, where wood, high in pitch and rosin, was freely introduced into the furnaces, in order that they might escape under cover of this smoke.
Early in the present war it was found that black smoke had a low obscuring power, showed frequent rents or holes and were difficult to standardize. Their production also caused a considerable loss in the speed of the vessel. They therefore fell into disuse except for emergency purposes and today the standard smoke for screening purposes of all kinds is, without exception, white.[33]
The properties most desired in a screening smoke, apart from low cost, are: (a) Maximum screening power, which refers to the question of density, i.e., a relatively thin layer must completely obscure any object behind it, and (b) Stability, which implies, among other things, a low rate of settling or dissipation. There is little reason to doubt that, within limits, the smaller the [Pg 286] particles of a smoke cloud, the more completely will the smoke possess these qualities. The screening power of a smoke cloud depends very largely upon the scattering of the light coming through it, and by analogy with those peculiar solutions which we call colloidal, we should expect the scattering to increase as the degree of subdivision increases, within limits. The rate of settling is unquestionably an inverse function of the size of the particles. The chief aim, therefore, in smoke production is to attain as high a degree of subdivision as possible. Methods may be classified as good or bad, in so far as they satisfy or fail to satisfy this criterion.
It is obvious that only gases or substances capable of being brought into the vapor state or into a very fine state of subdivision can be used for producing smoke clouds. The reaction product, of which the smoke particles consist, should preferably be:
(a) Solid. Otherwise the particles will tend to grow in size by condensation of the liquid particles present in the cloud.
(b) Non-volatile. If volatile, the particles will disappear by evaporation as the cloud is diluted by air currents. Larger particles will also form at the expense of the smaller ones.
(c) Non-deliquescent. If the particles are deliquescent, they will tend to grow by condensation of water vapor upon them.
(d) Stable towards the usual components of the atmosphere, especially moisture.
While it might seem that it would be difficult to fulfill these conditions, there are several chemical compounds which have been successfully used as smoke producers. This does not mean that they fulfill all the conditions, but they represent a compromise between the various requirements.
Phosphorus. One of the earliest materials to be used in smoke clouds was phosphorus. This is prepared on a commercial scale by heating phosphate rock (which contains calcium phosphate) with sand and coke in an electric furnace. Phosphorus occurs in two forms, white and red. White phosphorus, which is formed when the vapor of the [Pg 287] substance is quickly cooled, is, in the pure state, almost colorless, melts at 44° C., boils at 287° C., is readily soluble in various solvents, and is luminous in the air, at the same time emitting fumes (the oxidation product, phosphorus pentoxide). On gentle warming in the air, it takes fire and burns with a brightly luminous flame. Red phosphorus is obtained by heating white phosphorus out of contact with the air, to a temperature of 250° to 300° C. Red crusts then separate out from the colorless liquid phosphorus, and almost the entire amount is gradually converted into a red, solid mass. If this is freed by suitable solvents from the small amounts of unchanged white phosphorus, a dark red powder is obtained, which remains unchanged for a long time in the air, does not appreciably dissolve in the solvents for white phosphorus, does not become luminous, and can be heated to a fairly high temperature without igniting. Further, red phosphorus is not poisonous, while white phosphorus is highly so.
Either form burns to phosphorus pentoxide, which is converted by the moisture of the air to phosphoric acid,
4P + 5O₂ = 2 P₂O₅
2P₂O₅ + 6H₂O = 4H₃PO₄
Since one pound of phosphorus takes up 1.33 pounds of oxygen and 0.9 pound of water, it is not surprising that phosphorus is one of the best smoke producers per pound of material. Comparison of the value of the two forms for shell purposes have invariably pointed to the superiority of the white variety.
In addition to its use as a smoke producer, it is used in incendiary shell and in tracer bullets. For incendiary purposes a mixture of red and white phosphorus is superior.
Chlorosulfonic Acid. Chlorosulfonic acid, ClSO₂OH, was first employed by the Germans to produce white clouds, both on land and on sea. For this purpose, they sprayed or dropped it onto quicklime, the reaction between it and the lime furnishing the heat necessary for volatilization, though in this way about 30 per cent of the acid is wasted.
Chlorosulfonic acid is obtained from sulfur trioxide and hydrogen chloride, which combine when gently heated:
SO₃ + HCl = ClSO₂OH
[Pg 288]
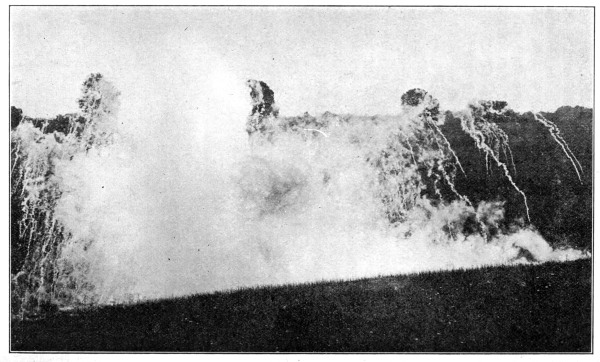
Fig. 86.—75 mm. White
Phosphorus Shell.
2 seconds after bursting.
[Pg 289] On a commercial scale, hydrogen chloride is passed into 20 per cent oleum, until saturation is reached. This is heated in a nitric acid still, when the chlorosulfonic acid distills over between 150°-160° C. With 30 per cent oleum, the conversion factor is about 42 per cent. The residue in the still is about 98 per cent sulfuric acid.
It forms a colorless liquid, boiling at 152° C., and having a density of 1.7.
Chlorosulfonic acid fumes in the air, because reaction with water forms sulfuric acid and hydrochloric acid.
ClSO₂OH + H₂O = H₂SO₄ + HCl
This material was not used by the United States since oleum was found superior.
Oleum. Oleum is a solution of 20 to 30 per cent sulfur trioxide (SO₃) in concentrated sulfuric acid. It has been used by the Germans to produce clouds on land and sea, by its contact with quicklime, and by the Americans for screening tanks and aeroplanes. Sulfur trioxide has been found to be superior as a shell filling. It is believed that the smoke producing power of oleum is due solely to its sulfur trioxide content, the sulfuric acid itself acting only as a solvent. The rather high freezing point of the oleum containing high percentages of sulfur trioxide is a disadvantage.
Sulfur Trioxide. Sulfur trioxide, SO₃, is a colorless mobile liquid, which boils at 46° C. and solidifies to a transparent ice-like mass, melting at 15° C. It is prepared by passing a mixture of sulfur dioxide and oxygen over finely divided platinum or other catalysts at a temperature between 400 and 450° C. Sulfur trioxide can only be used as a filler for shell and bombs, and is probably the best substitute for phosphorus.
Tin Tetrachloride. Tin tetrachloride, SnCl₄, is obtained by the action of chlorine on metallic tin. It is a liquid, boiling at 114° C., and having a density of 2.2. It fumes in the air, because it hydrolyzes to stannic hydroxide:
SnCl₄ + H₂O = Sn(OH)₄ + 4 HCl
It makes a better and more irritating smoke for shell and hand [Pg 290] grenades, than either silicon or titanium tetrachlorides. Since there is practically no tin in this country, the other tetrachlorides were developed as substitutes.
Silicon Tetrachloride. Silicon tetrachloride, SiCl₄, is prepared from silicon or from impure silicon carbide by heating it with chlorine in an electric furnace. The raw material (silicon carbide) is a by-product in the manufacture of carborundum. It is a colorless liquid, boiling at about 58° C., and fumes in moist air, owing to hydrolysis:
SiCl₄ + 4 H₂O = Si(OH)₄ + 4 HCl
It is not very valuable in shell, though it is more effective on moist, cool days than on warm, dry ones. Its greatest use is found in the smoke cylinder, combined with ammonia. By the action of the moisture of the air, the following reaction takes place:
SiCl₄ + 4 NH₃ + 4 H₂O = Si(OH)₄ + 4NH₄Cl
The addition of a lachrymator gives a mixture which works well in hand grenades for mopping up trenches.
Titanium Tetrachloride. Titanium tetrachloride, TiCl₄, is made from rutile, TiO₂, by mixing with 30 per cent carbon and heating in an electric furnace. A carbonitride is formed, which is said to have the composition Ti₅C₄N₄, but the actual composition may vary from this to the carbide TiC. This product is heated to 600-650° C., and chlorine passed through, giving the tetrachloride. It is a colorless, highly refractive liquid, which boils at about 136° C., is stable in dry air and fumes in moist air. The best smoke is produced by using 5 parts of water to one of the tetrachloride, instead of the theoretical 4 parts [which would form Ti(OH)₄.] Since it is more expensive to manufacture and not as effective as silicon or tin tetrachloride, it is used only as an emergency material.
Berger Mixture. One of the most important smoke materials was the zinc-containing mixture, which was used in the smoke box, the smoke candle, certain of the smoke grenades and in various forms of colored smokes. The basis of this was the Berger Mixture, which had the composition: [Pg 291]
| Zinc | 25 |
| Carbon tetrachloride | 50 |
| Zinc oxide | 20 |
| Kieselguhr | 5 |
This formula produced a light gray carbon smoke, with much carbon in the residue. In this mixture the zinc and carbon tetrachloride react to form zinc chloride and carbon; the kieselguhr keeps the mixture solid by absorbing the tetrachloride, while the zinc oxide is practically useless, as its absorbing power is small.
In order to accelerate the reaction and to oxidize the carbon, thereby changing the color of the smoke from gray to white, an oxidizing agent was added. Sodium chlorate was chosen for economic reasons. The reaction now proved to be too violent, and the zinc oxide was replaced by ammonium chloride. This cooled the smoke, retarded the rate of burning and added to the density of the smoke, since the obscuring power of the ammonium chloride is high. The kieselguhr was replaced by precipitated magnesium carbonate, which is as good an absorbent, gives a much smoother burning mixture, and also adds somewhat to the density of the smoke by virtue of the magnesium mechanically expelled. The mixture then had the composition:
| Zinc | 34.6 |
| Carbon tetrachloride | 40.8 |
| Sodium chlorate | 9.3 |
| Ammonium chloride | 7.0 |
| Magnesium carbonate | 8.3 |
In the problem of smoke production, the size of the particle is of great importance. Being a physical quantity it can easily be correlated with such physical properties as settling, diffusion, coagulation, and evaporation. These factors are more important in connection with toxic smokes, since there the penetration factor must be considered.
Smoke appears to consist of particles of all sizes from 10⁻³ cm., which [Pg 292] may just be resolved by the unaided eye, to molecular dimensions, 10⁻⁸ cm. The larger particles settle out most rapidly and so do not remain long in suspension.
Wells and Gerke have developed a form of ultra-microscope which is well adapted to the measurement of the size of smoke particles. The ultra-microscope is a low power microscope using intense dark ground illumination for viewing particles which are too small to be seen by transmitted light. They are rendered visible in this way, since any object, no matter how small, which emits enough light to affect the retina is visible, provided the background is sufficiently dark. Thus stars are visible at night and dust particles are easily seen in a sunbeam in a darkened room. The larger particles, viewed in this way, do not appear larger but brighter. The apparent size of the particles is determined by the diffraction pattern and is thus dependent only on the optical system used to view them. The more intense the incident light, the brighter the particles appear. In the ultra-microscope described, the image of an intense source, such as a concentrated filament lamp, or an arc, is focused upon the particles in the microscopic field, but the axis of the illuminating beam, instead of coinciding with the axis of the microscope, as ordinarily used, is perpendicular to it. The beam itself, therefore, never enters the microscope at all, but passes under the objective into a blackened chamber where it is absorbed. The field of the microscope is made dark by placing underneath the objective another “black hole” or blackened chamber with an opening just a little larger than the field.[34]
The method used for measuring the velocity consisted in causing the particle to describe a definite stroke many times in succession in an electric field. This was accomplished by reversing the direction of the field with a rotating commutator. The convection due to the source of light is perpendicular to this motion so that a zigzag line is obtained (see Fig. 88). The amplitude of this oscillation is an accurate measure of the distance traversed by the particle under the electric force for a definite small interval of time. The speed of the rotating commutator and the electric field are both susceptible of precise measurement, so that the size of a single particle is precisely determined. [Pg 293]
Fig. 87.—Ultramicroscope for Measuring Size of Smoke Particles.
[Pg 294]
Fig. 88.—Measurement of Smoke Particles by Use of Ultramicroscope.
[Pg 295] When a sample of smoke is viewed in the ultra-microscope, it appears like the starry heavens, except that the stars are dancing violently about. At first little distinction is made between the particles, as there seems to be no order in their motion, but soon it becomes evident that the brighter particles are more sluggish than the dim ones. This is due to the greater mass of the bright particles, for they are larger. The particles are all moving slowly away from the source of light and eventually diffuse to the walls of the cell.
When the electric field is turned on, about one-third of the particles immediately migrate, about equally in both directions, to the two electrodes. If the field is reversed, the direction of migration is reversed and if the commutator is used the particles oscillate regularly. Sometimes the particles may be seen to combine and become neutral, in which case oscillation ceases.
In measuring the concentration of smokes, the following terms are useful:
Density. The density of a smoke is defined as the reciprocal of the thickness of the smoke layer in feet necessary to obscure a given filament. Thus six inches of a smoke of density 2.0 is required to obscure an electric light filament, whereas one requiring four feet would have a density of 4. Another way to show the significance of this definition is to point out that if a definite weight of a stable smoke is diluted with air after it is formed, the product of the volume by the density always remains constant. Any marked variation in this rule may be taken as evidence that the particles of smoke are undergoing a change, in most cases due to evaporation.
Total Obscuring Power. The volume of smoke produced per unit weight of material used is the second factor in determining the value [Pg 296] of a smoke. The product of this volume per unit weight by the density of the smoke is the real measure of effectiveness, and is called the total obscuring power (T. O. P.) of the smoke. If the volume is expressed in cubic feet per pound and the density in reciprocal feet, the unit of T. O. P. is square feet per pound. That is, it expresses the square feet of a smoke wall, thick enough to completely obscure a light filament behind it, which could be produced from a pound of the reacting substances. The total obscuring power of some typical smokes are as follows:
| Phosphorus | 4600 |
| NH₄Cl(NH₃ + HCl) | 2500 |
| SnCl₄ + NH₃ + H₂O | 1590 |
| Berger Mixture | 1250 |
| SnCl₄ + NH₃ | 900 |
| SO₂ + NH₃ | 375 |
In all measurements of density, and therefore of T. O. P., the rate of burning must be considered. If a slow burning material be compared with a rapid one, the former will not reach its true maximum density, as a great deal of the smoke may settle out during the time of burning. Comparisons of T. O. P. are significant only when made on smoke mixtures of the same type and in about the same quantities.
Two methods of measuring the effectiveness of a smoke cloud have been devised, one, the smoke box, which measures the obscuring power directly by observing at what distance a lamp filament is obscured by intervening smoke, the other, the Tyndall meter, which measures the intensity of the scattering of the light.
The earliest measurements of smoke intensity are perhaps those of Ringelmann (Revue Technique, 19, 286), who devised the well known chart of that name, intended mainly for measuring intensities of black smoke issuing from a chimney at a distance. The first measurements for military purposes are probably due to Bertrand, who made numerous comparative studies with his “salle opacimetrique.” This was a room 23 × 14 × 3.6 meters, with 7 windows. Two doors, one [Pg 297] provided with 3 oculars 2 cm. in diameter, gave access to the room. On the other door, opposite the first, were hung several black signs. Six pairs of columns were spaced along the room at measured distances. When a smoke is produced in the room, the black paper signs first become invisible, then the door itself, and finally the columns, pair by pair. They reappear in the reverse order, and as a measure of relative opacity Bertrand took the time elapsing between the detonation and the reappearance of the farther door.
Fig. 89.—Tyndall Meter.
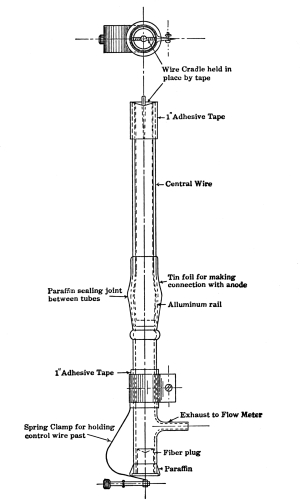
Fig. 90.—Cottrell Precipitation Tube.
Smoke Box. The smoke box, used by the C. W. S., was constructed of wood with tight joints, and had a moveable brass rod running through it to which was attached a small size 25-Watt Mazda lamp. The density [Pg 298] of each smoke introduced in the box is determined by moving this lamp back and forth until a point is reached when the pattern of the filament can just be distinguished by the observer looking in at the glass window, external light being excluded by a black cloth. The thickness of the smoke layer between the glass window and the light is recorded as the measure of the smoke density. For field tests, a larger box, 6 × 8 × 8 feet (288 cubic feet) was constructed. The observation light was moveable in a line connecting the mid-points of opposite sides of the box. To insure uniform distribution of smoke, a fan with [Pg 299] 18-inch blade revolved at any desired speed between 60 and 250 r.p.m. With this, results are obtained indicating both the original density and its stability.
Tyndall Meter. The Tyndall meter was first devised for studying smokes and mists. Tolman and Vliet adapted it to Chemical Warfare purposes, and used it in studying the properties of smokes.
The apparatus (Fig. 89) consists eventually of an electric light bulb, a condensing lens giving a beam of parallel light which passes through the diaphragm, and a Macbeth illuminometer for measuring the strength of the Tyndall beam. In case the material is a liquid suspension or solution, it is introduced into a cylindrical glass tube, while smokes and mists are premixed directly through the apparatus. The long closed tubes are provided, respectively, for absorbing the beam after it has passed through the disperse system and for giving a dark background for observing the Tyndall beam. Methods of standardization are given in the Journal of the American Chemical Society, 41, 299.
A third method for analyzing smokes consists in the use of an electrical precipitator. This apparatus consists essentially of a modified Cottrell Precipitator, with a central wire as cathode surrounded by a cylindrical foil as anode (Fig. 90). The smoke to be analyzed is drawn through the apparatus at a known rate, and the particles of smoke precipitated on the foil by means of a high voltage, direct current. The determination of concentration is made by weighing the foil before and after precipitation.
The smoke box was developed for the Navy for use when it was desirable to have the smoke screen generated away from the ship. (The smoke funnel, described later, was operated on board ship). The float consists of an iron container (holding the smoke mixture) surrounded by an iron float to support the apparatus when it is thrown into the water (Fig. 91). The iron container consists of a cylinder 22 inches high and 10 inches in diameter. One inch holes are bored 1½ inches from the top of this cylinder, from which the smoke is emitted. The iron float is about 2 feet in diameter and 8 inches deep. This box holds approximately 100 pounds of smoke mixture, and is so constructed that it will float one hour. When ignited, the mixture burns 9 to 9½ minutes. The smoke produced has a T. O. P. of about 1900. Fig. 92 shows the Navy Smoke Box in action. [Pg 300]
Fig. 91.—Navy Smoke Box.

Fig. 92.—Navy Smoke Box in Action.
[Pg 301]
Smoke candles are used for producing a cloud of smoke for screening purposes in or behind the lines. They are made by packing about three pounds of the modified Berger Mixture in a container (Fig. 93) (galvanized can 5¼ inches by 3½ inches) and are lighted by means of the match head type of ignition. Smoke is given off at a uniform rate for about 4 minutes, forming a dense, fog-like cloud which hangs low (Fig. 94). This smoke is absolutely harmless, and can be breathed without discomfort. The obscuring power is high and, with a favorable wind, a small number of the candles will produce a screen sufficiently dense to allow operations to be carried out unseen by the enemy.
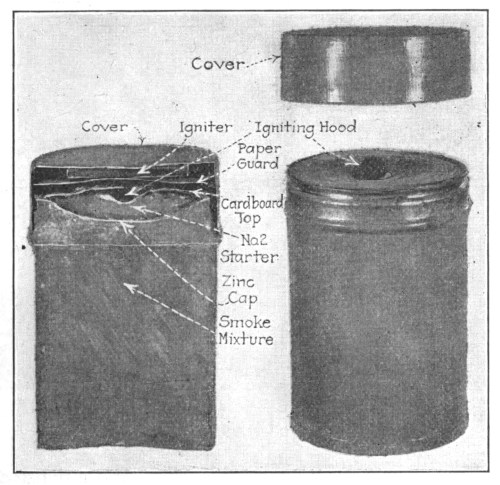
Fig. 93.—B. M. Smoke Candle.
[Pg 302]
The smoke grenade is also designed for use in trench and field warfare, where it is desired to produce a dense smoke screen. It is made by packing 340 grams of the standard smoke mixture in an ordinary light metal gas grenade. Around the top of the grenade are vents closed by a zinc strip. The ignition is caused by the standard bouchon when the grenade is thrown. The heat of the reaction burns through the zinc strip and a dense cloud of smoke is evolved for 45 seconds.

Fig. 94.—Smoke Cloud from B. M. Candle.
Stannic chloride has also been used extensively in hand grenades, as it gives a very disagreeable cloud of smoke upon detonation. Due to the high prices and urgent need of tin for other purposes, silicon tetrachloride was substituted for tin tetrachloride towards the close of the war. A mixture of silicon tetrachloride and chloropicrin was also used. This not only gives a very good smoke cloud, but combines with it the toxic properties of the chloropicrin cloud.
The method of firing the smoke grenade is the same as that of any [Pg 303] grenade using the same type of bouchon. Usually the grenade is grasped in the hand for throwing in such a manner that the handle of the bouchon is under the fingers. The safety clip is pulled out with the other hand and the grenade is thrown with an overhand motion. When the grenade leaves the hand, the handle of the bouchon flies off, allowing the trigger to hit the cap which ignites the fuse.
The white phosphorus combined hand and rifle grenade became the standard smoke grenade by the end of the war. Stannic chloride was used to clear out dugouts, but not as a smoke producer.
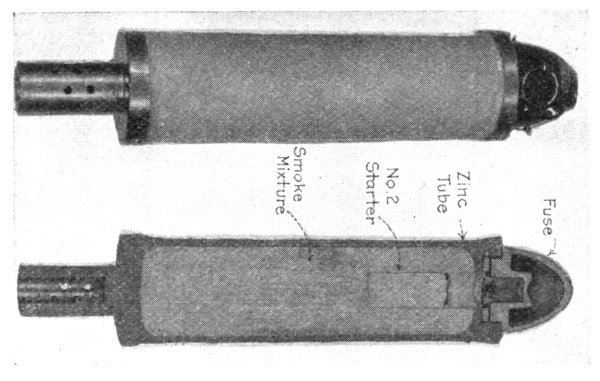
Fig. 95.—Stokes’ Smoke Shell.
The Stokes’ smoke shell was perfected to furnish a means of maintaining the best possible smoke screen at long ranges by means of an easily portable gun. The 3-inch Stokes shell, as adapted for combustion smokes, weighs about 13 pounds and contains about 4 pounds of standard [Pg 304] smoke mixture. This shell is designed to produce a continuous screen over a period of 3 to 4 minutes.
The Livens smoke drum was designed for use with the 8-inch Livens projector, so as to produce a smoke screen of large volume and long duration at long ranges. The drum, as adapted for combustion smokes, weighs 17.5 pounds empty and 49 pounds loaded. The smoke-gas mixture was specially adapted for use in the Livens drum.
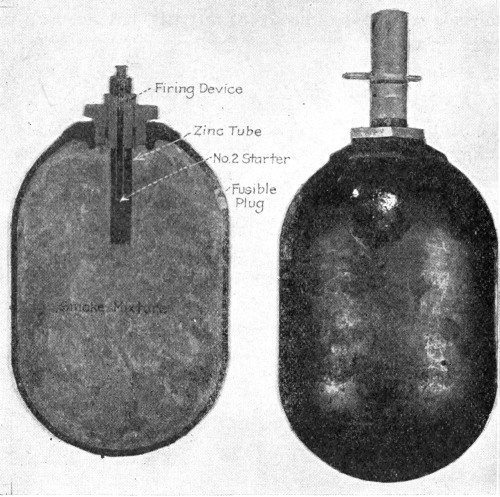
Fig. 96.—Livens Smoke Bomb.
Smoke mixtures in Livens were never used to any considerable extent in the war and it is questionable if they ever will be. A Livens can usually only be fired once before resetting, hence Stokes mortars are used whenever possible. [Pg 305]
The smoke funnel was developed for the production of a white smoke cloud from the stern of a vessel. The smoke producing materials are liquid ammonia and silicon tetrachloride, with carbon dioxide as a compressing medium. This is the most satisfactory compressing medium, because: (1) The silicon tetrachloride is forced out at nearly constant pressure. (2) The carbon dioxide is easily compressed to a liquid and can be handled in this form. Further, it has a vapor pressure of 800 pounds at 60° F., and a cylinder can be nearly emptied without loss in efficiency. (3) Carbon dioxide is sufficiently soluble in silicon tetrachloride to cause the latter to effervesce and thus materially aid in its evaporation on spraying. (4) Liquid carbon dioxide, behaving in a manner similar to liquid ammonia, affords a means for the silicon tetrachloride to “keep pace” with the ammonia, under changes in temperature, and thus ensures a more nearly neutral, and therefore the most effective, smoke.
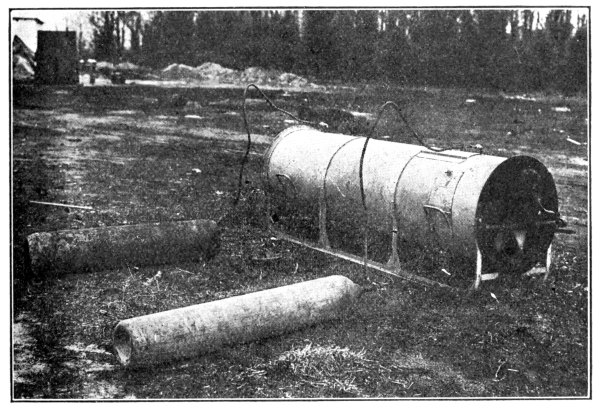
Fig. 97.—Navy Smoke Funnel.
[Pg 306] The smoke funnel proper consists of an open end cylinder, about 2 feet in diameter and 7 feet long, mounted in a horizontal position on an angle iron frame. At one end is an 18-inch fan securely fastened to the cross supports. This fan is operated by hand, through gears giving a ratio of about 30 to 1. The silicon tetrachloride enters the cylinder through a pipe, which terminates in four spray nozzles, while the ammonia enters through a single nozzle. The air forced into the funnel serves to hydrolyze the silicon tetrachloride and mixes the vapors. The resulting reaction evolves a dense white cloud of very large volume and high obscuring power. One set of cylinders is capable of maintaining this cloud for over 30 minutes. Under normal conditions the discharge is at the rate of 2 pounds of silicon tetrachloride to 1 pound of ammonia. To stop the smoke, the silicon tetrachloride is closed first, the ammonia allowed to run about half a minute, and the fan is shut off last.
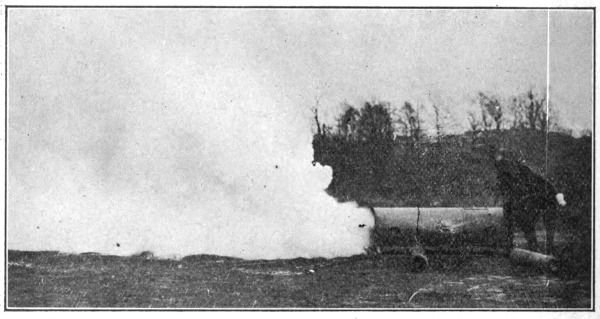
Fig. 98.—Navy Smoke Funnel in Operation.
The smoke knapsack furnishes a portable apparatus for smoke production. The gross weight is about 70 pounds; when in operation it gives a dense white smoke for about 15 minutes. The operation may be intermittent or continuous and the quantity of smoke is sufficient to completely hide [Pg 307] one platoon of men in skirmish formation with a 5-mile per hour enfilade wind. The apparatus consists of two steel tanks about 26 inches in height and 6 inches in diameter. From the side of each tank, but near the bottom, extends a short pipe on which is placed a suitable valve. A flexible armored hose connects the valve to a short length of pipe which is equipped with spray nozzle. The cylinders are charged with silicon tetrachloride and ammonia under pressure. The valves may be operated with the left hand, while the sprayer apparatus is held in the right. The release buckles are within easy reach of both hands.
While many special devices have been developed by means of which the gas troops and infantry are able to set up smoke clouds on short notice, the smoke shell, fired by the artillery, always played an important part in this work. In the same way that a large number of the poison gases were adapted to artillery use, so were most of the smoke producing substances.
As a filler for smoke shell, phosphorus easily ranks first, and is approached only by sulfur trioxide in very humid weather. A rough approximation to the relative values of some of its rivals is given in the following table:
| White phosphorus | 100 |
| Sulfur trioxide | 60-75 |
| Stannic chloride | 40 |
| Titanium chloride | 25-35 |
| Arsenic chloride | 10 |
Comparison of the value of different forms of phosphorus for shell purposes has invariably pointed to the superiority of the white variety. Mixtures of white and red (2 to 1) have also proved effective.
A complete barrage over a front of 200 yards can be established in from 40 seconds to 1 minute and maintained by firing a salvo followed by battery fire of 3 seconds. Four 4.5-inch howitzers could maintain an effective barrage over a front of 1000 yards. The influence of sunshine is very marked, as in moist, cool weather one shell every 15 seconds is sufficient. [Pg 308]
Fig. 99.—Smoke Screen for Tanks.
[Pg 309]
Tests have demonstrated (see Fig. 99) that successful smoke screens for tanks may be produced by spraying oleum into the exhaust. On a 7-ton tank of the Renault type (40 H. P.) 110 cc. per minute produced a large volume of smoke, which had excellent covering power, and which could be made intermittent or continuous at will.
The same method may be applied to aeroplanes, and to ships. It is calculated that a cylinder containing 300 pounds of 20 per cent oleum will maintain a smoke screen on a ship for a period of 15 minutes, if oleum is used at the rate of 23.6 pounds per minute. Since the cylinders may be arranged in batteries, the screen may be continued for any period of time. The Tank Corps rather favor phosphorus rifle grenades for producing a smoke screen at a distance from the tank.
Smoke screens may be employed with one or more of the following objects in view:
(1) To mask known enemy observation posts and machine gun nests; to conceal the front and flanks of attacking troops, concentration of guns and tanks, roads and concentration points; to blind the flashes of batteries in action and to hamper aerial observations.
(2) As a feint to draw the enemy’s attention to a front on which no attack is being made, so as to hold his troops to their trenches, or to induce him to expend ammunition needlessly and to put down a barrage in the wrong place.
(3) To simulate gas and force the enemy to wear his mask. Gas should occasionally be mixed with smoke, to impress upon him the belief that it is never safe to remain in a smoke cloud without wearing his mask.
(4) In rolling or mountainous country, to fill valleys with smoke and thereby conceal the advance from all observation, including aerial.
(5) To cover the construction of bridges, trenches, etc., in the face of the enemy. [Pg 310]
The pall of smoke that hung over every battlefield of the Civil War made a profound impression upon Fries when, as a boy, he first read of those battles. However, practically every reference made to smoke treated it as a nuisance. It obscured the field of vision and interfered with troop movements as well as with the aiming and firing of rifles and cannon, though due to their short range this was not so serious as it would be nowadays. Nevertheless so deeply was this interference appreciated that the most earnest efforts were made to discover a smokeless powder. This, as the world well knows, was developed with great efficiency during the latter part of the nineteenth century. With the development of the smokeless powders came also a better understanding of the action of powder, whereby the velocity of projectiles, and consequently the range and accuracy, were greatly increased. This increased range and accuracy of guns forced a consideration of protection,—and concealment is one form of protection.
The Navy would appear to have been the first branch of the American forces to realize how valuable a smoke screen may be. Thus Fries, in August, 1913, had the interesting experience of witnessing a week’s maneuvers at the eastern entrance to Long Island Sound between the Navy and the Coast Artillery. During that week the Navy carried out extensive experiments with smoke screens both by day and by night. The smoke in all cases was generated by smothering the fires on destroyers or other ships, thus causing dense clouds of black smoke to be given out from the funnels.
After the World War had been in progress some time and particularly about the time the United States entered it, a determined search was begun for more efficient smokes and more efficient smoke producers.
In the Navy, smoke screens were expected to be established by small craft behind which larger vessels could maneuver for position and range. These screens were also established for the purpose of cutting off the view of enemy submarines or other vessels, thus allowing merchant ships or even warships when injured or outclassed to escape. [Pg 311]
The Army was much slower to appreciate the value of smoke. In fact, apparently no army really realized the value of a smoke screen until after gas warfare became an accomplished fact. As is well known, the evaporation of the large quantity of liquid used in wave attacks caused a cloud of condensed moisture. This is what gave rise to the designation “cloud attack.”
English regulations for defense against gas in the early days called for every man and animal to stand fast upon the approach of a gas cloud and remain quiet until the cloud had passed. Thus casualties were reduced to a minimum and the English were fresh to receive the attack that was frequently launched immediately after the cloud had passed. The Germans finally thought of the plan of sending over a fake gas attack. In that way they simply produced a smoke cloud that looked like a gas attack. Naturally the English stood fast as before. The Germans attacking in the fake cloud naturally caught the British at a complete disadvantage with consequent disastrous results to the latter.
But that was a game at which two could play. About this time the value of white phosphorus for producing a smoke screen was taken up by the British and large numbers of 4-inch Stokes mortar shells were filled for that purpose. All armies then began to experiment with smoke producing materials. Most of these were liquid. Of them all, as has been stated before, white phosphorus, a solid, proved the best. Toward the close of the war these smoke screens began to be used to a considerable extent for the purposes given above. No one who has engaged in target practice and encountered a fog, or who has hunted ducks and geese in a fog needs to be told of the difficulty of hitting an object he cannot see.
The First Gas Regiment proved its worth and won everlasting glory by using the Stokes’ mortars of the British with their phosphorus bombs for attacking machine gun nests. The white phosphorus in that case had a double effect. It made a perfect smoke screen, thereby making the German machine gun shots simply shots in the dark, while at the same time the burning phosphorus forced the gunners to abandon their guns and surrender. Thus phosphorus played and will play in the future the [Pg 312] double rôle of forming a defensive screen and of viciously attacking enemy troops. This phosphorus, which catches fire spontaneously, burns wet or dry, total immersion in water alone sufficing to put it out. This means of extinguishing the flames being almost totally absent on the battlefield, it can be truthfully said that burning phosphorus is unquenchable. The burns are severe and difficult to heal. For these reasons white phosphorus will be used in enormous quantities in any future war.
All armies have begun to realize this value of smoke. In the future it will be the infantryman’s defense against all forms of weapons and it will be used on every field of battle, by every arm of the service and at all times, day or night. It is even more effective in shutting out the light from searchlights, star bombs and similar illuminants for use in night attacks than it is in daylight. With this straight use of smoke for protection will go its use along with poisonous gases. Every smoke cloud will be poisonous or non-poisonous at the will of the one producing the cloud, and this will be true whether it is produced from artillery shell, mortar bombs, hand grenades, smoke candles or other apparatus. Thus smoke and gas together will afford a field for the exercise of ingenuity greater than that of all other forms of warfare. The only limitation to the use of smoke and gas will be the lack of vision of commanders and the ignorance of armies.
Proper recognition and aid given to chemical warfare development and instruction in peace are the only methods of overcoming these limitations. In this, as in all other development work, the most serious obstacle comes from the man who will not see, whether it be from a lack of intelligence, laziness or inbred opposition to all forms of advancement.
[Pg 313]
The introduction of diphenylchloroarsine as a poison gas really introduced the question of toxic smokes. This material, as has already been pointed out, is a solid, melting at about 30°. In order to secure efficient distribution, the material was mixed with a considerable amount of high explosive. When the shell burst, the diphenylchloroarsine was finely divided or atomized and produced a cloud of toxic particles. Since smoke particles are only slightly removed by the ordinary mask, this formed a very effective means of chemical warfare.
An analogous result was obtained by the use of poison gases, such as chloropicrin, in a smoke cloud produced from silicon or stannic chloride. Here, however, the toxic material was a real gas, and so the real result attained consisted in forcing the men to wear their masks in all kinds of clouds. The true toxic smoke went further in that the ordinary mask offered little protection and thus compelled the warring nations to develop a special type of smoke filter.
These smoke clouds consist of very small particles, which may be considered as a dispersed phase, distributed in the air, which we may call the dispersing medium. The dispersed phase may be produced by mechanical, thermal, or chemical methods.
Mechanical dispersion consists in the tearing apart of the material into a fine state of subdivision. It may be called a hammer and anvil action. The more powerful the mechanical force, the smaller the resulting particles. This may be accomplished by the use of a high explosive, such as the Germans used in the case of diphenylchloroarsine.
The production of smoke by thermal dispersion depends essentially upon the fact that when a substance of sufficiently low vapor pressure is volatilized, and the vapors are passed into the air, [Pg 314] they recondense on the nuclei of the air to form a smoke. Vaporization from an open container, permitting the vapors to pass directly to the air without being quickly carried away from the surface of evaporation, produces smoke having larger particles, because each particle formed remains for an appreciable period of time in contact with air saturated with vapor, and hence grows very rapidly.
The easiest way to produce small smoke particles is to mix the toxic material directly with some fuel which will produce a large amount of heat and gas upon burning. When this mixture is enclosed in a container having a small orifice, upon burning, the toxic vapor and gas will pass through this orifice at high velocity; it has been demonstrated by Lord Rayleigh that the size of the particles depends upon the velocity of emission of the gas from a given orifice.
The product of chemical combination may include a super-saturated vapor, which condenses into small particles.
Explosive dispersion is really a combination of mechanical dispersion followed by thermal dispersion.
The fundamental idea underlying all the work on toxic smokes is to obtain a smoke that has marked penetrating power. Screening power is not important here. In addition to penetration, a smoke should be highly toxic and have a slow rate of settling.
Penetration may be tested by the use of a standard filter; a suitable filter for this purpose is one which does not remove the smoke to such an extent that measurement of its concentration becomes difficult, and one which does not become clogged quickly by the smoke. A filter consisting of two pads of felt, placed side by side and arranged so that the smoke first comes in contact with the thinner and less dense pad has been found very satisfactory.
In testing penetration, smoke is produced by dispersing one gram of the toxic substance in a sheet iron box of 1000 liters capacity. After 5 minutes a steady concentration is usually attained and the smoke is [Pg 315] then forced through a Tyndall meter, (see page 299) after dilution with air, where the initial concentration is determined. It then passes through the standard filter, and through a second Tyndall meter, where the final concentration is measured. The difference of the two readings gives the amount of smoke retained by the filter. The penetration is ordinarily represented by a series of figures, which decrease from a maximum value at the beginning of the test to a minimum at a point where the filter permits the passage of so little smoke that it cannot be measured. This decrease is due to decrease in penetrating power and concentration of the smoke, and to increase in filtering power of the filter as a result of plugging. Usually five degrees of penetration are recognized, excellent, good, fair, poor and very poor.
Fig. 100.—Penetration Apparatus Used to Test Toxic Smokes.
A portable penetration apparatus is shown in Fig. 100. In using the apparatus, the smoke producing material is so placed with reference to the apparatus that the sample is taken about 20 feet down the wind, so that the smoke is appreciably diluted. One man is stationed at each [Pg 316] Tyndall meter and takes readings as fast as his recorder can write them, so that the smoke density, before and after the filter, can be followed very closely.
In addition to a high penetrating power a smoke should also possess great toxic, irritant, sternutatory, or lachrymatory power. These properties are tested by exposing mice to the smoke in the chamber. They are placed in the chamber at the beginning of the run, and exposed for 10 minutes to the smoke from 1 gram of the material. While these tests are only qualitative in character, they give a fairly good notion of the relative value of different materials.
It has been found that, if the optical readings from the Tyndall meter are plotted as ordinates against the time t (the time elapsed after detonation) as abcissas, and that portion of the curve between t = 0 and t = 30 considered, the curve generally descends sharply at first, from a high point representing the density immediately after the production of the smoke, to a point in the neighborhood of t = 8, where it flattens out and descends much more slowly with a slope that changes little. The area under the significant portion of the curve, that is, the area circumscribed by the curve from the point t₃₀ to t₀, the vertical axis from this point to the origin, the horizontal axis from the origin to t₃₀ and the line perpendicular to this axis, cutting the curve at t₃₀, is a rough measure of the relative values of different smokes. This area is calculated as the sum of two rectangles, from t₀ to t₈ and from t₈ to t₃₀.
Some results are as follows:
| Area 30 | |
| Phenyldichloroarsine | 181 |
| Triphenyldichloroarsine | 178 |
| Diphenylcyanoarsine | 137 |
| Diphenylchloroarsine | 101 |
| Cyanogen bromide | 94 |
| Methyl dichloroarsine | 70 |
| Phenylimidophosgene | 69 |
| Mustard gas | 38 |
[Pg 317] The curves in Fig. 101 show the way in which the readings fall off with time. Each substance of course has its characteristic curves.
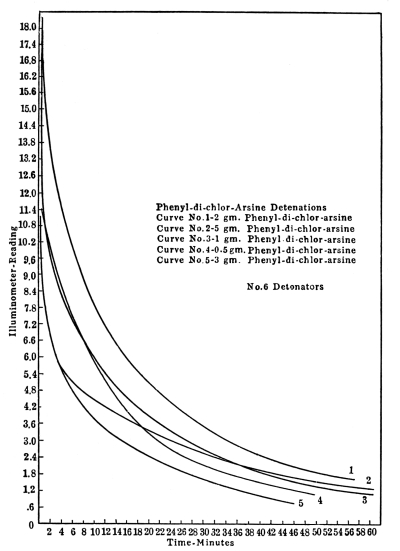
Fig. 101.—Typical Curves
Showing the Decrease
in Concentration of Smoke Cloud with Time.
The selection of materials for the production of toxic smokes can only be carried out experimentally. A number of very toxic substances have been shown to be valueless as toxic smokes because of low penetration, decomposition during the process of smoke production, or for other reasons. [Pg 318]
Arsenic compounds produce smokes distinctly better than the average. Inorganic compounds which have high melting and boiling points are very poor smoke producers. The only exception to this is magnesium arsenide, which may suffer decomposition. Compounds like mercuric chloride and arsenic tribromide, which boil or sublime at comparatively low temperatures, produce good smokes. Most materials which boil below 130° C. produce no smoke as they evaporate on dispersion. It is difficult to set any upper limit for the boiling point beyond which materials do not produce good smokes, but in all probability 500° C. is not far from the maximum. Liquids and solids are, on the whole, almost equally good as smoke producers. The physical condition of the material has no great effect upon the amount of smoke which it will produce. This seems to depend only upon the physical and chemical properties of the material.
It has been mentioned above that the Germans used a shell, containing solid diphenylchloroarsine and a high explosive. A 10.5 cm. shell (Blue Cross) was about two-thirds filled with cast trinitrotoluene and contained a glass bottle with 300-400 grams of toxic material. Diphenylchloroarsine was also used in shell, in solution, a mixture of phosgene and diphosgene (superpalite) being the ordinary solvent (Green Cross). Mixtures of diphenylchloroarsine and phenyldichloroarsine were also used.
In the case of high explosive shell, the use of a separate container appears to be desirable, because a mixture with the explosive seriously decreases its sensitiveness and even its destructive power. There is also a question as to the stability of such a mixture. However, 75 mm. shell containing 30 per cent diphenylchloroarsine mixed with T. N. T. gave good clouds of toxic smoke.
Two toxic smoke candles were developed by the Chemical Warfare Service, known as the B-M Toxic Smoke Candle, perfected by the Pyrotechnic Section of the Research Division, and the Dispersoid Smoke Candle, developed by the Dispersoid Section. [Pg 319]
The B-M Toxic Smoke Candle consists of a bottle-shaped sheet steel toxic container set into a can, containing smoke mixture. The heat from the burning mixture causes the distillation of the toxic material. The toxic vapor is discharged through a nipple, screwed into the neck of the container and extending over the top of the smoke can. Steel wool is used in the toxic container to reduce the violent boiling and spattering of the material. A small amount of steel wool, held in place by a wire screen, is also used in the nipple for the same purpose. The toxic container is sealed by a fusible metal plug, melting at 90° C., cast into a retainer at the base of the nipple. The fusible plug melts upon the first application of heat and allows free passage of the vapor into the smoke cloud. The ignition of the apparatus is effected by means of a simple match head and an accompanying scratcher.

Fig. 102.—Toxic Smoke Cloud from 500 D. M. Candles.
The candles were placed in 5 parallel rows which were 2 yards apart, each row containing 100 candles on a 100 yard front. The total time of active smoke emission was 23 minutes.
The first evolution of smoke occurs about 10 seconds after the first appearance of flame. About one minute after ignition the toxic material [Pg 320] will begin to distill into the smoke cloud and this will continue for about four minutes. The burning of the candle should be complete in about six minutes.

Dispersoid CandleBritish Candle
Fig. 103.—Comparison of Dispersoid and British D. M. Candles.
The Dispersoid Toxic Smoke Candle differs from the B-M candle in that the toxic container is not used. A mixture of smokeless powder and the toxic material (diphenylchloroarsine or D. M., an arsenical obtained from arsenic trichloride and diphenylamine) is filled directly into the container, a cylindrical can 3.5 inches in diameter and 9 inches high made from 27 gauge sheet metal, and packed under a total pressure of 2,500 pounds. The top of the candle is a metal cover, containing the match head scratcher, which is separated from the match head by a Manila paper disc. These are the same as those used in the B-M candle. The candle has a total weight of about 4.25 pounds, of which 3.6 pounds are the smoke mixture, containing about 1.3 pounds of toxic material.
In operating the candles, the cover is removed and the match head ignited by friction with the scratcher. The match head burns through the cardboard and ignites the powder. The heat and gas produced by the combustion of the powder vaporizes the particles of the toxic material and carries the vapors out through the orifices at a high velocity whereupon they recondense to form a smoke. The rapid emission of the vapors through the orifice prevents any possibility of their ignition. [Pg 321]
The time before good emission of smoke takes place after the ignition of the match tip of a candle is 30 seconds. The average time of vigorous smoke emission is from four to five minutes. The result of a field test with the dispersoid candle is shown in Fig. 102. A comparison of a British and a Dispersoid candle is shown in Fig. 103. It should be stated that this may not have been a fair test as only one British candle was available for the comparative test.
[Pg 322]
The first types of the Standard Box Respirator contained cotton pads, which sufficed to remove the ordinary smoke of the battlefield and even that from the earlier toxic materials. Improved methods of producing toxic smokes, by means of which smaller particles were obtained, led, early in 1918, to the recognition of the need of improved protection against these smokes. The first attempts to meet this need consisted in improving the filtering qualities of these pads. It was soon found, however, that to make better filter pads would greatly increase the total resistance of the canister. This was highly undesirable, since the resistance of the ordinary canister was already so high as to be very uncomfortable. To overcome this objection, some of the early designs of filter canisters were provided with a mechanical valve, which could be operated by hand, to by-pass the air around the filter when the canister was used against gas alone, or so set as to make the air pass through the filter when smoke was feared. This introduced a factor of uncertainty among the men during a gas attack, since each man must decide for himself whether smoke was present. This reason alone was sufficient for discarding this design.
A preliminary study of the situation indicated that any filter for fine smoke particles must have a high resistance per unit of area, but that the total resistance must be comparatively low. In order to secure the large area necessary to bring the total resistance within reason, the experimental work was developed along three lines: The formation of a filter into a bag, cup, or jacket to surround the outside of the canister; the use of an arrangement sufficiently compact to go inside [Pg 323] the canister; and the use of a filter as a separate unit, to be attached to the canister by an air connection.
A survey of the possible filtering materials indicated that only two offered promise, namely, paper and felt.
Reports that the British had developed thin, creped, sulfite-cellulose wood pulp paper for filters led to an intensive study of this material by the Chemical Warfare Service.
Fig. 104.—Crepe Paper Doughnut Filter Canister.
In general we may say that the development of paper filters (in sheet form) met with little success. Papers affording the required protection did not live up to the resistance specifications. The reason for this probably is in the method of making paper. The pulp is fed onto the screen of a Fourdrinier machine under conditions that do not permit of uniformity in the distribution of the fibers and consequently there is no uniformity in the size of pores. In order to eliminate the large [Pg 324] holes, which allow the smoke to pass readily, the paper must be pressed to reduce these pores to the proper magnitude. This naturally results in an approximately equal decrease in the size of the small pores, with a consequent increase in the final resistance out of all proportion to the protection gained. A very satisfactory paper was finally produced, but the resistance was too high and it was necessary to increase the total available filtering area, which resulted in the accordion type of filter. This filter was incapable of development on a large scale because of the large amount of hand work required in assembling. The lack of uniformity in a single sheet has been overcome with some success by making up a filter from 40 to 80 layers of tissue or crepe paper, trusting that the law of chance would bring the large pores in some successive layer. Such a filter was adopted by the British, but since it did not give protection comparable with that afforded by felt filters, it was rejected in the United States.
In the so-called “doughnut” filter use was made of tissue paper. Instead of seeking for uniformity in a vertical direction through a block of tissues, it was sought along the axis horizontal with the sheet. The effectiveness of such a filter was less than that of felt. In addition, serious difficulty was met in cutting the pile of tissue paper into the proper shape so that eventually it was abandoned as a production possibility.
Work on the felt filters started about June, 1918. Great difficulties were met in the beginning, as a felt satisfactory for this purpose must be made under carefully controlled conditions and production conditions during the war did not readily lend themselves to such control. However, the opportunities afforded in felt making for uniform packing and arranging of the fibers (the whole process of making a felt, is one of gradual packing of fibers into a relatively small volume) are such as to assure a greater degree of success than is the case in paper making.
Very successful filters have been obtained with the use of felt. There are two serious objections to its use, however. The first is the great [Pg 325] cost of the filter (this was above one dollar per filter at the close of the War); the second is that felt is a valuable industrial commodity. It is thus very desirable that a cheaper and a less important industrial material be found.
Just before the Armistice, the Gas Defense Long Island Laboratory brought out the so-called “1919 Canister,” which consisted of an oval section, perforated metal, war gas material container with a central, flat, perforated breathing tube connected to a nozzle at one end. (See also page 228.) After this inner container is packed with the war gas chemicals, a filter jacket is slipped over it and the top edge sealed to the inner container.
Fig. 105.—1919 Felt Filter Canister.
[Pg 326] Attempts were made to put paper filters on this canister by wrapping it with layers of paper. In some cases, layers of coarse burlap or mosquito netting were applied between the layers of paper to give mechanical strength and air space. The fact that many filters gave good protection showed that a filter of this type and material is possible, but the operations of wrapping and sealing require careful work in production and inspection and even with the greatest skill and care, imperfections are almost impossible to avoid. This chance of defects, together with the labor involved, makes the process undesirable.
Tolman, Wells and Gerke, during the course of their work on toxic smokes, developed the following theory of smoke filters.
The phenomena occurring in the filtration of smoke are exceedingly complicated, but the general nature of the process may be simply described in terms of the kinetic properties of the small particles comprising the smoke.
A filter may be regarded as a series of minute capillaries through which the smoke slowly flows. In order that filtration may take place, it is not necessary to assume that the capillaries of the filter are smaller than the particle, for the particles may diffuse to the walls of the capillaries and it is believed that with typical filters this is the actual method of smoke removal for particles less than 10⁻⁴ cm. in diameter.
In accordance with this view as to the nature of smoke filtration, the important factors involved are (1) the Brownian motion of the smoke particles, (2) the area and arrangement of the internal surface presented by the filter, (3) the flow of the smoke as a whole, and (4) the attractive forces between the filter surfaces and the smoke particles. The first three of these factors determine how many particles come within the range of the mutual forces of the particle and filter surface, and the fourth factor determines the chance or expectation that the particle will permanently adhere to the surface of the filter. [Pg 327]
All the early tests made on smoke filters used diphenylchloroarsine, because it was felt that the filter must be tested against a toxic smoke. A man test was developed as representative as possible of actual conditions in the field, and the time necessary for a man to detect diphenylchloroarsine smoke in the effluent stream when breathing at a normal rate, using a carefully controlled concentration of smoke produced by detonation, was used as the criterion of the protection offered by the canister. This test was subject to extensive individual variations, due to the varying physiological resistances of different men to diphenylchloroarsine smoke. Further, it was quite inadequate for rapid testing on a large scale. A testing machine was then developed, which gave results comparable with those obtained in the man test. The method used in detecting the gas was physiological, that is, by smell or by its irritating action towards the membranes of the eye. While these are purely qualitative tests, they are much more sensitive than any possible chemical tests.
Because of the desirability of having a method which could be controlled chemically, other methods were developed.
Ammonium chloride is a solid smoke, consisting of particles of quite variable sizes. It is sensitive to dilution and clogs the pores of the filtering medium quite rapidly. For this reason it was used in the study of the rate of plugging or clogging of the filter (the closing of the pores of the fabric or other material to the passage of air).
The smoke is produced by the reaction of ammonia and hydrogen chloride-air streams. The smoke thus generated is passed from the mixing chamber to a larger distribution box and from there through the filter, at a standard rate. The concentration of the smoke may be accurately determined by chemical means or photometrically, using a Hess-Ives Tint Photometer, the Marten Photometer, or a special photometer developed by the Chemical Warfare Service.
A comparison of a large number of tests with those of other smokes [Pg 328] would indicate that ammonium chloride smoke offers accurate information as to protection sought, but is hardly a desirable smoke for testing on a large scale.
The third method developed was the sulfuric acid smoke. This smoke was produced by passing dry air through a tower of solid pieces of sulfur trioxide and then mixing the vapor with a large volume of air at 50 per cent relative humidity. It is not a clogging smoke and the filtering efficiency does not change materially in the time of exposure required for a test. The smoke lends itself easily to chemical analysis and offers data as to exact particulate cloud concentrations which will penetrate canisters; photometric measurements are also applicable.
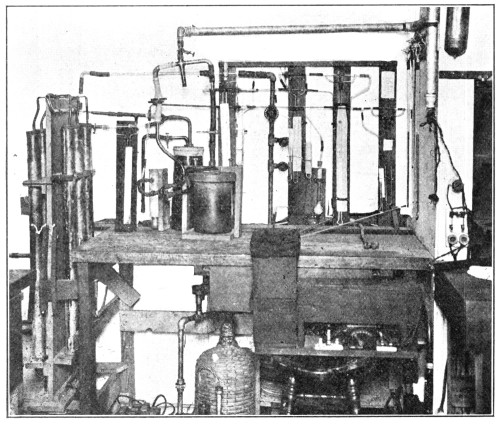
Fig. 106.—Tobacco Smoke Apparatus for Testing Canisters.
The fourth method consists in the use of tobacco smoke. This is generated by passing air over ignited sticks of a mixture of tobacco [Pg 329] (63 per cent), rosin (30 per cent) and potassium nitrate (7 per cent). This smoke is composed of particles of extreme uniformity in size; chemically it is relatively inert. It is not a clogging smoke and is not sensitive to moisture and dilution. The density of the effluent smoke is compared with that of the entering smoke in a Tyndall beam, and the filtering capacity of the material determined in terms of the amount of air necessary to dilute the entering air to the same concentration of the effluent air. The method is simple in manipulation and the test is a rapid one (50 canisters per day). Because of the apparent superiority of tobacco smoke as a testing smoke, the accompanying disadvantages are possibly outweighed.
From the standpoint of inherent chemical properties, the general desirability of a suitable testing smoke would decrease in the following order: tobacco, sulfuric acid, ammonium chloride.
[Pg 330]
The success of pyrotechnics in night signalling led, during the World War, to considerable attention being paid to the development of pyrotechnic signals for day use. This was mainly directed to the production of distinctive smokes, which should have the same long range visibility under varying light conditions. Since a gray or white smoke might be confused with the smoke produced accidentally by the explosion of shell, it was necessary to use smoke of definite and unmistakable colors, and red, blue, yellow, green and purple smokes were developed. During the early part of the war, only a yellow smoke was in use, though others were added later.
There are three possible ways of obtaining signal smokes.
I. The first method, while possible, can never be an efficient method of producing signals. Some success was met with in dispersing certain inorganic materials, as rouge, and ultramarine, in projectiles fired from a 3-inch mortar and exploded by a time fuse arrangement at the height of their flight. Various mixtures were also tried, such as antimony oxysulfide and aluminum powder (red), arsenic and antimony trichlorides with sodium thiosulfate (yellow), etc., but these compositions have the disadvantages of being liable to catch fire if dispersed by a black powder explosion.
II. While colored smokes may be produced by chemical reaction, such [Pg 331] as the combination of hydrogen iodide (HI), chlorine and ammonia, the clouds are not satisfactory as signals. In this particular case, the purple cloud (to the operator in the aeroplane) appeared white to the observers on the ground.
High temperature combustion smokes have also been studied. These are used in the so-called smoke torches. The yellow arsenic sulfide smoke is the most widely used. Most formulas call for some sulfide of arsenic (usually the native realgar, known commercially as “Red Saxony Arsenic”), sulfur, potassium nitrate, and in some cases, a diluent like ground glass or sand. A typical mixture consists of:
| Red arsenic sulfide | 55% |
| Sulfur | 15% |
| Potassium nitrate | 30% |
A very similar smoke may be obtained from the following mixture:
| Sulfur | 28.6% |
| White arsenic | 32.0% |
| Potassium nitrate | 33.8% |
| Powdered glass | 6.6% |
These smokes are not as satisfactory in color as the smoke produced by a dye smoke mixture, especially when viewed from a distance, with the sky as a background. They fade out rather quickly to a very nearly white smoke.
A black smoke upon first thought might seem to be the easiest of all smokes to produce, but actually the production of a black smoke that would be satisfactory for signalling purposes was rather a difficult matter.
Starting with the standard smoke mixture, which gives a white or gray smoke, hexachloroethane, which is solid, was substituted for the carbon tetrachloride, in order to avoid a liquid constituent. Naphthalene was first used, until it was found that the mixture of naphthalene and hexachloroethane melted at temperatures below that of either of the constituents. Anthracene was then substituted. The principal reaction is between the magnesium and the chlorine-containing compound, whereby magnesium chloride and carbon are formed. The reaction is very violent, and a white smoke is produced. The anthracene slows down the reaction [Pg 332] and at the same time colors the smoke black. The speed of the reaction may be controlled by varying the anthracene content.
In burning this type of smoke mixture in a cylinder, it is essential that free burning be allowed. It has been found that if combustion is at all smothered, and the smoke forced to escape through a comparatively small opening, it will be gray instead of dense black.
III. Various attempts have been made to utilize the heat evolved when the Berger type smoke mixture reacts to volatilize or mechanically disperse various colored inorganic substances, and especially iodine. These were unsuccessful. Modifications, such as
| Strontium nitrate | 1 part |
| Powdered iron | 2 parts |
| Iodine | 3 parts |
were also tried, but while such mixtures ignited easily, burned freely and evenly, and gave a continuous heavy purple cloud, they were very sensitive to moisture and capable of spontaneous ignition.
The most satisfactory and successful colored smokes are those produced by the volatilization of organic dye materials. This practice seems to have originated with the British, who produced such smokes by volatilizing or vaporizing special dyes by igniting mixtures of the dye, lactose and potassium chlorate and smothering the combustion.
In selecting dyestuffs for this purpose it was at once recognized that only those compounds can be used which are volatilized or vaporized without decomposition by the heat generated when the mixture is ignited and the combustion smothered. It was also found that the boiling point and melting or volatilization point of the colored compound must be close enough together so that there is never much liquid dye present. Since all colored organic compounds are destroyed if subjected to sufficient heat, the mixture must be so prepared and the ignition so arranged that the heat generated is not sufficient to cause this destruction.
The oxidizing agents used in the combustion mixture may be either potassium or sodium chlorate. The nitrate is not satisfactory. Lactose [Pg 333] has proven the best combustible. Powdered orange shellac is fairly satisfactory but offers no advantage over lactose.
The following dyes have been found to give the best smokes:
| Red | “Paratoner” |
| Yellow | Chrysoidine + Auramine |
| Blue | Indigo |
| Purple | Indulin (?) |
| Green | Auramine Yellow + Indigo |
At the beginning of the war, the only colored smoke used by the United States Army was a yellow smoke. The smoke mixture used in all signals, excepting the smoke torch, was the old arsenic sulfide mixture. The following smoke signals were adopted during the World War:
| Signal Parachute Rocket | Yellow and Red |
| V. B. Parachute Cartridge | Yellow |
| 25 mm. Very Parachute Cartridge | Yellow |
| 35 mm. Signal Cartridge | Yellow |
| 35 mm. Signal Cartridge | Red |
| 35 mm. Signal Pistol | |
| 25 mm. Very Signal Pistol | |
| V. B. Rifle Discharger Cut |
From the days when Horatius kept the bridge, down through the centuries to the World War, all leaders in battle were pictured at the front and with flaming sword, mounted on magnificent chargers, or otherwise so prominently dressed that all the world knew they were the leaders. During all these hundreds of years commands on the field of battle were by the voice, by the bugle, or by short range signals with arms, flags, and swords. Even where quite large forces were involved they were massed close enough ordinarily so that signalling by such means sufficed to cover the front of battle. In those cases where they did not, reliance was put upon swift couriers on horseback or on foot.
With the invention of smokeless powder and the rifled gun battles were begun and carried on at greater and greater ranges. Artillery fired not [Pg 334] only 2,000 to 3,000 yards but up to 5,000 and 10,000 yards, or even, as in the World War, at 20,000 yards and more. It was then that other means of signalling became essential. Distant signalling with flags is known to have been practiced to a certain extent on land for a long time. The extension of the telegraph and telephone through insulated wires laid by the Signal Service was the next great step in advance, and in the World War there came in addition the wireless telephone both on land and in aeroplanes and balloons.
Along with this development, as mentioned under Screening Smokes, came the development of the use of smoke for protection and for cutting off the view of observers, thus making observation more and more difficult. This use of smoke, coupled with the deadly fire of machine guns and high explosives, forced men to take shelter in deep shell holes, in deep trenches and other places that were safe, but which made it nearly impossible to see signals along the front of battle.
Every man can readily be taught to read a few signals when clearly indicated by definite, sharply defined colored smokes. At first these were designed for use on the ground and will be used to a certain extent in the future for that purpose, particularly when it is desired to attract the attention of observers in aeroplanes or balloons. In such cases a considerable volume of smoke is desired. For the man in the trench or shell hole some means of getting the signal above the dust and smoke of the battlefield is needed. It is there that signal smokes carried by small parachutes, contained in rockets or bombs, have proven their worth. These signals floating high above the battlefield for a minute or more, giving off brilliantly colored smokes, afford a means of sending signals to soldiers in the dust and smoke of battle not afforded by any other method so far invented. As before stated, every man can be taught these simple signals, where but very few men can be taught to handle even the simplest of wireless telephones.
Thus, smoke has already begun to complicate, and in the future will complicate still more, every phase of fighting. It will be used for deception, for concealment, for obscuring vision, for signalling and to hide deadly gases. The signal rocket will be used to start battles, change fronts, order up reserves, and finally to stop fighting. [Pg 335]
The signal smokes by day will be displaced at night by brilliantly colored lights which will have the same meaning as similarly colored smokes during the day. Thus, literally, smoke in the future will be the cloud by day and the pillar of fire by night to guide the bewildered soldier on the field of battle with all its terrors and amidst the confusion, gas, smoke and dust that will never be absent while battles last.
[Pg 336]
Since it is generally known that white phosphorus, when exposed to the air, takes fire spontaneously, it logically follows that numerous suggestions should have been made for using this material in incendiary devices. Practice, however, has shown that, while phosphorus is undoubtedly of value against very easily ignitable materials, such as hydrogen in Zeppelins, or the gasoline tanks of aeroplanes and dry brush or grass, it is of much less value when wood and other materials are considered. This is partly because of the low temperature of burning, and partly because the product of combustion (phosphoric anhydride) is really an excellent fireproofing substance. In view of this, phosphorus was used primarily for smoke production.
A superior incendiary material is found in thermit, a mixture of aluminum and iron oxide. When ignited, it produces an enormous amount of heat very quickly, and the molten slag that results from the reaction will prolong the incendiary action upon inflammable materials. When used alone, however, it has the disadvantages that the incendiary action is confined to a small area and that the heat energy is wasted because of the fact that it is so rapid in its action.
For this reason it is customary to add a highly inflammable material, which will become ignited by the thermit and will continue the conflagration. Petroleum oils, carbon disulfide, wood distillation products and other inflammable liquids were thoroughly tested for this purpose. The final conclusion was reached that oil, solidified with soap (sodium salts of the higher fatty acids) by a special method developed by the Chemical Warfare Service, was by far the best material to be used. In certain tests, using a combination of thermit and solid oil, flames fifteen feet high were obtained, which would be very useful against walls, ceilings, etc. [Pg 337]
In addition to this type of incendiary material, it was desirable to have a spontaneously inflammable mixture of oils, which could be used in Livens’ shell, Stokes’ shell or aeroplane bombs. The basis of these mixtures is fuel oil and phosphorus. By varying the proportions of the constituents it is possible to obtain a mixture that will ignite immediately upon exposure to the air, or one that will have a delayed action of from 30 seconds to two minutes.
The incorporation of metallic sodium gives a mixture that will ignite when spread upon water surfaces.
The incendiary devices used during the late war included: bombs, shell, tracer shell and bullets, grenades, and flame throwers.
Incendiary bombs were used almost exclusively by aircraft. The value of bombs which would cause destruction by starting conflagrations was early recognized but their development was rather slow. While the designs were constantly changing, two stand out as the most favored: a small unit, such as the Baby Incendiary Bomb of the English, and a large bomb, such as the French Chenard bomb or the American Mark II bomb.
In general bombs which, when they function upon impact, scatter small burning units over a considerable area, are not favored. Small unit bombs can be more effectively used because the scatter can be better regulated and the incendiary units can be more advantageously placed.
German Bombs. Incendiary bombs were used by the Germans in their airplane raids, usually in connection with high explosive bombs. A typical armament of the later series of German naval airships consisted of the following:
| 2 | 660-pound bombs |
| 10 | 220-pound bombs |
| 15 | 110-pound bombs |
| 20 | Incendiary bombs |
making a total weight of about 2½ tons. [Pg 338]
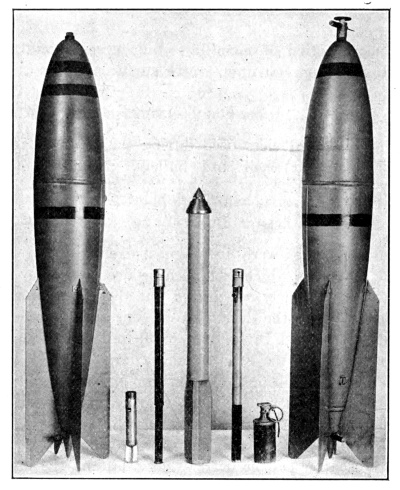
Fig. 107.—Incendiary Devices.
(From Left to Right).
Mark II Bomb, B. I. Bomb, Mark I Dart, Mark II Dart,
Mark I Dart, Grenade, Mark I Bomb.
A typical German bomb is shown in Fig. 108. It consists essentially of a receiver of white iron (R) composed of a casing and a central tube of zinc, joined together in such a fashion that, when the whole was complete, it had the appearance of an elongated vessel with a hollow center. Within this central hollow is placed a priming tube (T) of thin sheet iron, pierced by a number of circular openings. The receiver is about 445 mm. (17.5 in.) high and 110 mm. (4.3 in.) at its maximum diameter. It is wrapped with strands of tarred [Pg 339] cord over nearly its entire length. The empennage (270 mm. or 10.6 in.—in height) consisted of three inclined balancing fins, which assured the rotation of the projectile during its fall.
In the body of the bomb was a viscous mass of benzine hydrocarbons, while the lower part of the receiver contained a mixture of potassium perchlorate and paraffin. The central tube apparently contained a mixture of aluminum and sulfur.
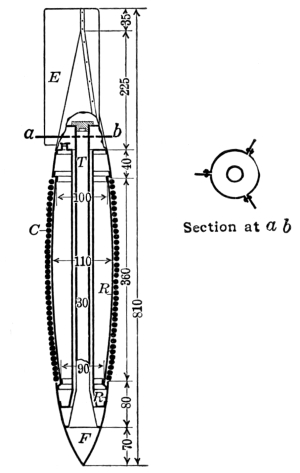
Fig. 108.—Aerial Incendiary
Bomb,
November, 1916.
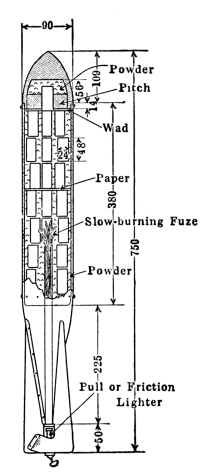
Fig. 109.—German Incendiary
Bomb,
Scatter Type.
All dimensions in millimeters.
Later the Germans used a scatter type of bomb (Fig. 109) which was designed to give 46 points of conflagration. Each of these 46 small cylinders contained 50 grams of an air incendiary material. They were arranged in layers, packed in with very fine gun powder. The bomb is ignited by a friction lighter which is pulled automatically when the bomb is released from the aeroplane. The bomb is constructed to burst in the air and not on striking the ground. The upper part of the projectile consists of a cast iron nose riveted to the sheet iron body of the bomb. When the explosion occurs, the nose is blown away and the small incendiary cylinders are scattered in the air. [Pg 340]
The incendiary material appears to be a mixture of barium nitrate and tar. Its incendiary power is very low because combustion takes the form of a small flame of very short duration. It should, however, be very valuable for firing inflammable materials.
British Bombs. The early British bombs were petrol bombs, which were used without great success for crop burning. Phosphorus bombs were then used for attacking aircraft. But the most successful incendiary is the so-called “Baby Incendiary Bomb.” This is a 6.5-ounce bomb with an incendiary charge of special thermit. These small bombs are carried in containers holding either 144 or 272 bombs. The former container approximates in size and weight one 50-pound H.E. bomb and the latter one 120-pound H.E. bomb. The bomb contains a cartridge very much like a shot gun shell which, on impact, sets down on the striker point in the base of the body, and causes the ignition of the charge. It is claimed that the cartridge of the B.I. bomb burns when totally immersed in any liquid (water included) and in depths up to two feet the flame breaks through the surface.
French Bombs. The French used three types of incendiary bombs, a special thermit (calonite), the Chenard and the Davidsen. The Chenard bomb is a true intensive type and is thought to be very successful. It functions by means of a time fuse operated by the unscrewing of a propeller, before striking the ground, and reaches its target in flames. Its chief disadvantage is the small amount of incendiary material which it carries. The Davidsen bomb expels its charge as a single unit and is not considered as valuable or as successful as the Chenard.
American Bombs. The program of the Chemical Warfare Service included three types of bombs:
| Mark II | Incendiary drop bomb |
| Mark III | Incendiary drop bomb |
| Mark I | Scatter bomb |
Mark II Bomb. The incendiary Mark II drop bomb is designed to be dropped from an aeroplane and is intended for use against buildings, etc., when penetrating effect followed by an intensive incendiary action is sought. [Pg 341]
The bomb case consists of two parts: a body and a nose. The body is a tapering zinc shell which carries the firing mechanism and stabilizing tail fin at the small end and at the large end a threaded ring which screws into the nose. The nose is of drawn steel of such shape as to have low end-on resistance and is sufficiently strong to penetrate frame structures.

Fig. 110.—Loading Bombs with “Solid” Oil.
The incendiary effect is produced by a thermit charge carried in the nose of the bomb. This charge is ignited by a booster of “Thermit Igniter” fired by black powder. The latter is ignited by a flash from [Pg 342] the discharge of a standard 0.30 caliber service cartridge contained in the body of the bomb, and exploded by a firing mechanism of the impact type. This method of firing has proven wholly unsatisfactory and will be superseded by some more direct-acting mechanism. The body of the bomb is filled with solidified oil. The molten thermit burns through the case of the bomb and liberates the oil which has been partially liquefied by the heat of the thermit reaction. Additional incendiary effect is afforded by the sodium nitrate contained in the nose below the thermit, and by two sheet lead cylinders filled with sodium and imbedded in the solid oil. The sodium increases the difficulty of extinguishing the fire with water.
Mark III Bomb. This bomb is simply a larger size of the Mark II bomb, its weight being approximately 100 pounds as compared with 40 pounds for the Mark II bomb. It is designed to be dropped from an aeroplane and is intended for use against buildings when marked penetrating effect is desired. The method of functioning is the same as the Mark II bomb and it has the same defects in the firing mechanism.
Mark I Bomb (Scatter Type). The Mark I incendiary drop bomb is also designed to be dropped from aeroplanes and is intended for use against grain fields, ammunition dumps, light structures or similar objectives when only a low degree of ignition is required. It is of the so-called scatter type, due to the action of the exploding charge which casts out incendiary material within a radius of 20 feet from the point of contact.
The incendiary action is due to the ejection of the various incendiary units in the bomb by the explosion of the black powder in the nose. The flash of this explosion serves to ignite the units. A powder charge in the rear of the bomb acts simultaneously with the nose charge, opening the bomb casing, and aiding materially in the scatter of the units. The bomb is so arranged as to function close to the ground, which is a further factor in the scatter of the units.
The incendiary units are waste balls about 2.5 in. in diameter and having an average weight of 2.5 ounces, tied securely with strong twine. These are soaked in a special oil mixture. Carbon disulfide and [Pg 343] crude turpentine, or carbon disulfide, benzene heads and crude kerosene gave satisfactory results. A later development attempted to replace the waste balls by solid oil, but the difficulties of manufacture and questions of transportation argued against its adoption.
These bombs were not used at the front. Nearly all of the American incendiary bombs proved too light on the nose and lower half, generally resulting in deformation upon impact and very poor results. New ones will be made stronger.
The British early recognized the value of a small bomb, and consequently adopted their B.I.B. (Baby Incendiary Bomb), weighing about 6.5 ounces. These are capable of being dropped in lots of 100 or more and thus literally shower a given territory with fire. The intensity of fire at any given point is much less than that obtained with the larger bombs, but the increased area under bombardment more than counter balances this disadvantage. While the British aimed at the perfection of a universal bomb, the American service felt that two classes should be developed, one to be used against grain fields and forests, the other against buildings.
The first class was called the Mark I Dart. This consisted of an elongated 12-gauge shot gun shell, filled with incendiary material and provided with a firing mechanism to ignite the primer as the dart strikes the ground. The flash of the primer sets fire to the booster, which, in turn, ignites the main incendiary charge. The latter burns several minutes, with a long flame. A retarding stabilizer attached to the tail of the dart serves the two-fold purpose of insuring the functioning of the firing mechanism and, by retarding the final velocity of the dart, preventing the collapse of the dart body when dropped from very high altitudes.
The incendiary mixture is one which gives a long hot flame, burns for several minutes and leaves very little ash. In general it consists of an oxidizing agent (barium or sodium chlorate), a reducing agent (aluminum, or a mixture of iron, aluminum and magnesium), a filler (rosin, powdered asphaltum or naphthalene) and in some cases a binder (asphaltum, varnish or boiled linseed oil). [Pg 344]
The Mark II Dart was developed to furnish a small size penetrating agent. It consists of a two-inch (diameter) zinc case filled with thermit and solid oil as the incendiary materials and provided with a cast iron nose for penetration. During the first half minute after firing, a pool of molten iron is formed by the thermit, which is very penetrating and affords a good combustible surface for the oil, which burns for an additional ten minutes.
It has an advantage over the Mark I dart in that it penetrates, and over the Mark II bomb in that it is smaller and lighter in weight.
Incendiary shell have been successfully used against aircraft and to some extent in bombardments of inflammable ground targets. Anti-aircraft shell are of small caliber and are usually tracer-incendiary. Such shell are filled with pyrotechnic mixtures which ignite at the moment of firing, or by time fuse, and are effective against highly inflammable material. Shell filled with thermit which explode and scatter the molten iron have been used against aircraft and ground targets, but with rather poor results. Large shell, which burst upon impact and scatter units of burning materials, have been used with some success against ground targets.
Tracer shell contain such mixtures as barium nitrate, magnesium and shellac, or red lead and magnesium.
Incendiary bullets are only effective against highly inflammable material, and are therefore used principally in aerial warfare against aircraft, either for the purpose of igniting the hydrogen of the gas bag, or the gasoline. The present tendency is towards the use of the large size (11 mm.) bullet, because of its greater incendiary action.
The incendiary material is either white phosphorus or a special incendiary mixture consisting of an oxidizing agent and some [Pg 345] combustible or mixture of combustibles. The white tracer bullet contains a mixture of barium peroxide and magnesium. A red bullet contained in addition, strontium nitrate and chloride, or peroxide.
While the use of incendiary grenades and other small incendiary devices is limited, such armament is considered very valuable in trench warfare. They can be used to set fire to inflammable material, either in offensive or defensive operations.
Phosphorus grenades, while used principally for producing smoke (see page 302), have considerable value as an incendiary weapon.
Thermit grenades are very useful in rendering unserviceable guns and other metallic equipment which must be abandoned. They permit aviators to destroy planes which motor troubles oblige to land in enemy territory. They are also used to ignite inflammable liquids, thrown into a dugout, or sprayed over an objective by a flame projector.
The Mark I hand grenade was developed for burning enemy ammunition dumps, for clearing away brush or other material in front of trenches and for use in dugouts. The standard H.E. grenade body was half-filled with thermit and half with a celluloid container filled with a solidified fuel oil. The grenade is fired by the spit of the fuse of the bouchon firing mechanism. This, through the booster, lights the thermit igniter, which in turn fires the main charge of thermit. The resulting molten iron readily penetrates the grenade case, at the same time igniting the celluloid case and its contents. The oil burns for about 3.5 minutes. This grenade was never used since it was considered that an all thermit grenade would be of more value.
Special projectiles were designed for use with the Stokes mortar and the Livens’ projector. Thermit was used only in Stokes’ projectiles. [Pg 346] The Livens’ projectile was filled with inflammable units (chlorated jute) immersed in a light oil mixture. Thrown from a projector into the enemy’s trench, it explodes, giving a large flash and scattering the burning units over an area of forty yards. The Mark II projectile, designed for general incendiary effect against readily inflammable material, consists of an altered 8-inch Livens’ gas projectile filled with chlorated jute units impregnated with solid oil and immersed in a spontaneously inflammable oil. After a short delay, these units burst into flame and burn vigorously for several minutes. It is almost impossible to extinguish them without large quantities of water. Such bombs have only a very limited use, so that it is questionable if they are really worth while.
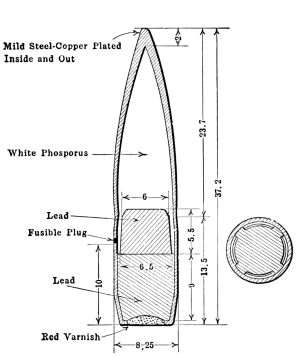
Fig. 111.—German 8"
Incendiary Bullet.
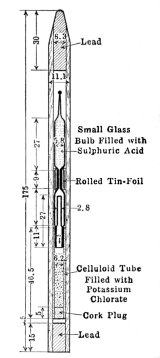
Fig. 112.—German Incendiary
Blue Pencil.
A very interesting and curious device was developed by the Germans in [Pg 347] the form of an incendiary pencil. Similar in appearance to a common blue pencil, sharpened at one end, they are distinguished only by a small, almost imperceptible, point placed on the outside 11 mm. from the unsharpened end. They are 175 mm. long, 11.1 mm. in diameter and weigh 12 to 13 grams. The interior of the pencil contains a glass bulb, with two compartments filled with sulfuric acid and a celluloid tube filled with potassium chlorate. The glass bulb ends in a slender point; when this is broken the acid comes into contact with the chlorate and causes an explosion. The two materials are separated by a layer of clay, which causes a delayed action of about 30 minutes. The operator breaks the point of bulb, buries the pencil vertically in the inflammable material and then has half an hour in which to get away, before any possibility of a fire. He cuts the pencil with a knife 2 cm. from the point, so that if caught he has the appearance of simply sharpening a pencil.
Among the unsuccessful weapons of the late war, the liquid fire gun or Flammenwerfer, as the German called it, is probably the most interesting. Its origin, according to a German story, was due to a mere accident. A certain officer, during peace maneuvers, was ordered to hold a fort at all cost. During the sham fight, having employed all the means at his disposal, he finally called out the fire brigade and directed streams of water upon the attacking force. Afterwards, during the criticism of the operations in the presence of the Kaiser, he claimed that he had subjected the attackers to streams of burning oil. The Kaiser immediately inquired whether such a thing would be possible, and was assured that it was entirely feasible. [Pg 348]

Fig. 113.—Liquid Fire Attack.
Long series of experiments were necessary before a satisfactory combination of oils was produced, which could be projected as a flame on the enemy, but they were finally successful. Unlike the use of poison gas, however, the flaming liquid gun did not prove to be a successful weapon of warfare. True, at first they were rather successful, but this was before the men learned their real nature. In the first attack, the Allies were completely surprised and the troops were routed by the flames. Auld tells of one of the early attacks (July 29, 1915) when, without warning, the front line troops were enveloped in flames. Where the flames came from could not be seen. All that the men knew was that they seemed surrounded by fierce, curling flames, which were accompanied by a loud roaring noise, and dense clouds of black smoke. Here and there a big blob of burning oil would fall into a trench or saphead. Shouts and yells rent the air as individual men, [Pg 349] rising up in the trenches or attempting to move in the open, felt the force of the flames. The only way to safety appeared to be to the rear. This direction the men that were left took. For a short space the flames pursued them and the local retirement became a local rout. After the bombardment which followed, only one man is known to have returned.
After a study of the pictures of the liquid fire gun in operation, it is evident that the men could not be blamed for this retirement. One has only to imagine being faced by a spread of flame similar to that used for the oil burners under the largest boiler, but with a jet nearly 60 feet in length and capable of being sprayed round as one might spray water with a fire hose.
Later, when the device was better known it was different, though even then it was a pretty good test of a man’s nerve. It was found that the flames could not follow one to the bottom of a trench as the gas did, and that, if a man crouched to the bottom of his trench, his head might be very warm for a minute or so but that the danger was soon past and he then could pick off the man who had so recently made things uncomfortable for him.
While it is said that Major R., who invented the Flammenwerfer, enjoyed a great popularity among his men, and is familiarly known as the Prince of Hades, there is no doubt that this was not shared by the Allies. Their rule was: “Shoot the man carrying the apparatus before he gets in his shot, if possible. If this cannot be done, take cover from the flames and shoot him afterwards.”
The German had several types, which may be grouped into the small or portable and the large Flammenwerfers.
The portable Flammenwerfer consisted of a sheet steel cylinder of two compartments, one to hold compressed nitrogen, the other to hold the oil. The nitrogen furnished the pressure which forces the oil out through the flexible tube. Air cannot be used, because the oxygen would form an explosive mixture with the vapors of the oil, and any heating on compression, or back flash from the flame or fuse might make things very unpleasant for the operator. A pressure of about 23 atmospheres is [Pg 350] reached when the cylinder is charged. The nitrogen appeared to be carried on the field in large containers and the flame projectors actually charged in the trenches.
The oil used in the flame projectors varied from time to time, but always contained a mixture of light or easily volatile and heavy and less volatile fractions of petroleum or mineral oil, very carefully mixed. In some cases even ordinary commercial ether has been found in the cylinders.

Fig. 114.—Small Flammenwerfer.
The most interesting part about the flame projector is the lighting device. This is so made that the oil ignites spontaneously the minute the jet is turned on, and is kept alight by a fiercely burning mixture which lasts throughout the discharge. This mixture is composed of barium nitrate, potassium nitrate, metallic magnesium and charcoal, with some resinous material. The priming consists of black powder and metallic magnesium.
When the oil rushes out of the jet, it forces up the plunger of a friction lighter and ignites this core of fiercely burning mixture. [Pg 351]
The range of these small projectors is from 14 to 17 meters (17 to 20 yards) but the duration of the flame is rather less than a minute.
In a later pattern, it was designed that one nozzle should be issued to three reservoirs. After the discharge of one, the jet is attached to the others in succession. This is called the “Wx” Flammenwerfer (interchangeable). In this way a squad of three men could carry 58 pints of inflammable oil. It is a question, though, whether the third man would live to use his reservoir.
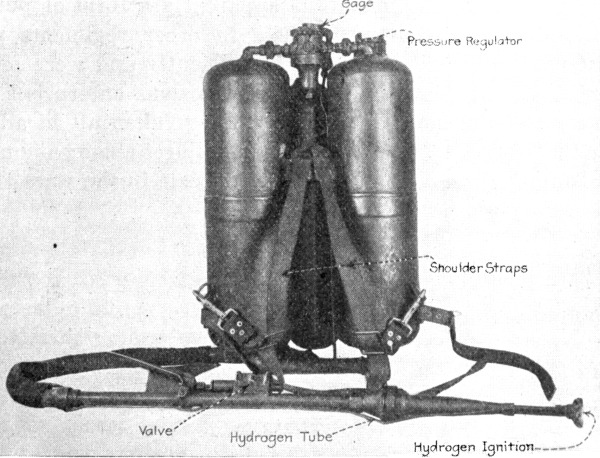
Fig. 115.—Boyd Flame Projector.
The fact that the trenches were often very close together during the early part of the war, made possible the use of large or stationary Flammenwerfer. These consisted of a steel reservoir 3⅓ feet in height and 1⅔ feet in diameter, weighing about 250 pounds, which could be connected to two steel cylinders, containing nitrogen under pressure. These carried 180 liters (40 gallons) of liquid and operated under a pressure of 15 atmospheres. The discharge nozzle was at the end of a metal tube three feet long, and its orifice was about ⁵/₁₆ of an inch [Pg 352] in diameter. The range of this apparatus was from 33 to 40 yards and the duration of the flame from one to two minutes. Because of the comparatively short range of these guns and the ease with which they could be destroyed, if located by the enemy, their use was very limited.
Even with the portable flammenwerfer, the most difficult thing to do is to get near enough the target to make the shoot effective. Another serious disadvantage is its very short duration. It is impossible to charge up again on the spot, and the result is that once the flame stops, the whole game is finished and the operators are at the mercy of the enemy.
With these facts in mind it is easy to see how service in the flaming gun regiments is apparently a form of punishment. Men convicted of offenses in other regiments were transferred either for a time or permanently and were forced under threat of death in the most hazardous enterprises and to carry out the most dangerous work. Taken all in all the flame thrower was one of the greatest failures among the many promising devices tried out on a large scale in the war.
[Pg 353]
The pharmacology of war gases plays such an important part in chemical warfare that a brief discussion may well be given of the methods used in the testing of gases for toxicity and other pharmacological properties.
War gases may be divided into two groups: persistent and non-persistent, each of which may include several classes:
| I. | Lethal |
| II. | Lachrymatory |
| III. | Sternutatory |
| IV. | Special |
Each class necessitates special tests in order to determine whether or not it is suitable for further development.
One of the first points which must be carefully determined in investigating a substance is its toxicity. It is important that this be determined for numerous reasons:
1. To determine what concentrations are dangerous in the field.
2. To ascertain how effective protective devices have to be to furnish sufficient protection against the gas.
3. To furnish a basis for accurate experimental work on the treatment of gassed cases.
4. To decide whether or not the material is worthy of further development in the laboratory or in the plant.
These considerations necessitate the determination of the toxicity in the form of a vapor and not by the ordinary method of administration by mouth, through the skin (subcutaneously) or through the blood (intravenously). The simplest method of determining the toxicity of a substance as a vapor would be to place animals in a gas-tight box and introduce a known amount of the substance in the form of vapor. But by [Pg 354] this method the concentration is not accurately known unless chemical analyses of the air are made, and then it is found to be much less than that calculated from the amount of substance introduced, because of condensation on the walls of the chamber, or absorption of the substance by the skin and hair of the animal and in some cases, of decomposition of the substance by moisture in the air. Moreover, it is found that the concentration decreases markedly with time. Because of these factors, the figures used for the concentration are more or less guess work. To overcome these difficulties, a chamber is used through which a continuous current of air, containing a known and constant amount of the poisonous vapor, is passed. Such an apparatus is shown in Fig. 116.
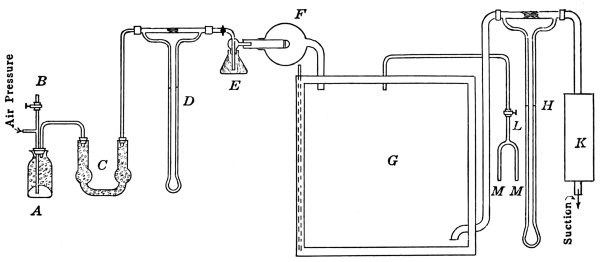
Fig. 116.—Continuous Flow Gassing Chamber for Animals.
The flask E is a 300 cc. Erlenmeyer flask, with a ground glass stopper. The liquid to be tested is placed in this flask together with a sufficient quantity of glass wool to prevent splashing and the carrying over mechanically of droplets of the liquid. Air is passed through A and C (calcium chloride drying tubes) and the rate measured by the flow meter D. The air and gas are mixed in F before passing into the chamber G. This chamber is made of plate glass, is of about 100 liters capacity, and is air-tight. The entire flow of air and gas through the box, kept constant at 250 liters per minute, is measured at H. The gas is removed through K, which is filled with charcoal and soda-lime, in order that little gas may pass into the pump.
By weighing the flask E, and its contents before and after [Pg 355] passing air through it, and knowing the total volume of the mixture passing through the chamber during the same period, the concentration of the substance can readily be calculated. This concentration, as determined by the “loss in weight” method, can be checked by chemical analysis (samples taken at M—M). The method has been found to give accurate values.
The concentration in the chamber reaches its constant level within 30 to 40 seconds after the apparatus is started.
With the flow of 250 liters per minute, the difficulties mentioned above are reduced to a point where they are practically negligible.
All toxicity tests on mice were made with an exposure of ten minutes, while dogs were exposed for thirty minutes. In case death did not occur during exposure, the animals were kept under observation for several days. Toxicity and all other figures are expressed in milligrams per liter of air, though parts per million (p.p.m.) was frequently used during the early work.
Another point of difficulty is the great individual variation in the susceptibility of animals. This is probably greater than when the poison is administered subcutaneously or intravenously. It necessitates the use of a large number of animals in making a determination of the toxicity of a gas. Again, the toxicity for different species may vary, and as the ultimate aim is a knowledge of the toxicity for man, a great many different species must be used. If the toxicity is widely different for different animal species, it is hard to arrive at a definite conclusion as to the toxicity for man.
With longer exposures than thirty minutes the lethal concentration is usually less, there being a cumulative effect. This is not true for hydrocyanic acid. If the concentration is not enough to kill at once, an animal can stand it almost indefinitely. Whether the action is cumulative or not depends on the rate at which the system destroys or eliminates the poison. If the poison is being eliminated as fast as received the concentration in the tissues cannot increase. It is stated, for example, that the amount of nicotine in a cigar would kill a man if taken in one dose. If it is spread over twenty minutes, the destruction or elimination of the nicotine is so rapid that no obviously bad effects are noted. [Pg 356]
Another interesting thing about the work on poison gases is that in most cases a preliminary exposure to less than the lethal concentration does not seem to make the animal either more or less sensitive on a later exposure. This is quite unexpected, because we know that with irritating gases, especially lachrymators, men adapt themselves to much higher concentrations than they could stand at first. In view of the experiences of arsenic eaters, it is quite possible that the experiments, which showed no accustoming to toxic gases, were not continued long enough to give positive results.
Not only does the susceptibility of different animals of the same species vary greatly for a particular gas, but the susceptibility of different species varies greatly with different gases. Thus while the effects of certain gases on mice are quite comparable to the effects on man, it is very far from being true with other gases.
While one cannot determine the lethal concentrations of poison gases for men, it is possible to determine the concentration that will produce lachrymation. The threshold value is that at which two-thirds of the observers experience irritation. The lachrymatory value is considerably higher than the threshold value.
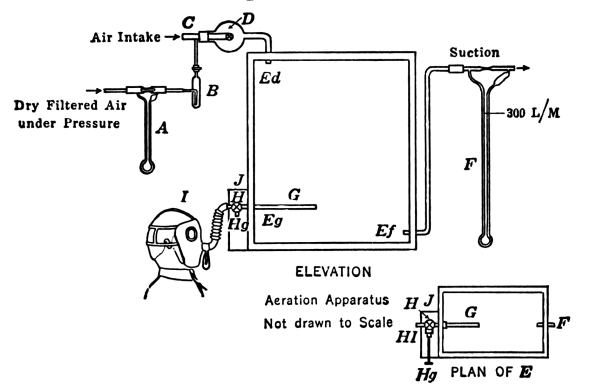
Fig. 117.—Aeration Apparatus for Testing Lachrymators.
A very satisfactory method for determining lachrymatory values is shown in Fig. 117. Air is measured at A and bubbled through [Pg 357] the lachrymatory substance in B. The air and gas are mixed in D and pass into E, a gas-tight, glass-walled chamber of about 150 liters capacity. The gas is removed through Ef, by suction and the volume of the air-gas mixture measured by the flow meter, F.
After the apparatus has run a few minutes, and the concentration of the gas has become constant, the subject is instructed to adjust the mask, attached at H, and to tell whatever he notices just as soon as he notices it. The operator stands in such a position that he can manipulate the stopcock H without being observed by the subject. After breathing air for a time (H is a two-way cock, connected with the air through J, and to the chamber through Eg) both to become accustomed to the mask and to eliminate, as far as possible, any “psychological symptoms,” the subject is allowed to breathe the gas mixture for a maximum of three minutes. If the expected symptoms are produced in less than this time, the test is discontinued as soon as they develop.
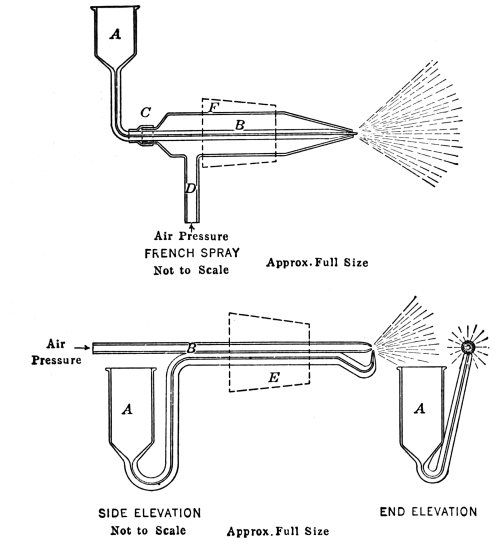
Fig. 118.—Type of Spray Nozzles.
[Pg 358] For accurate work, it is necessary to work with a pure sample which is at least fairly volatile. Mixtures cannot be run by this method. In this case it is necessary to volatilize each separately, passing the vapors simultaneously into the mixing chamber E.
A spray method may also be used with satisfactory results. Types of sprays are shown in Fig. 118.
Because of the great value in detecting low concentrations of gases in the field, it is important to know the smallest amount of a gas that can be detected by odor. In some cases, this test is more delicate than any chemical test yet devised.
Odors may be divided into two classes, true odors, and mild irritation. By true odor is meant a definite stimulation of the olfactory nerve, giving rise to a sensation which is more or less characteristic for each substance producing the stimulation. Mild irritation defines the sensation which is confused with the sense of smell by untrained observers, but which is really a gentle stimulation of the sensory nerve endings of the nose. This so-called odor of substances producing this effect is not characteristic. Higher concentrations of these compounds almost invariably cause a definite irritation of the nose.
Examples of true odors are the mercaptans, mustard gas, bromoacetone, acrolein, chlorine and ammonia. Substances which cause mild irritation are chloroacetone, methyl dichloroarsine, ethyl iodoacetate and chloropicrin.
In making the test for odor, the same apparatus is used as for lachrymators. The time of exposure is shortened to 30 seconds, as the subject always detects the odor at the first or second inhalation.
In this connection the recent work of Allison and Katz (J. Ind. Eng. Chem. 11, 336, [1919]) is of interest. They have designed an instrument, “the odorometer,” for measuring the intensity of odors in varying concentrations in air. It is based on the principle given above. A measured volume of air is passed through the liquid and then diluted to a given concentration. The mixture is then passed through a rubber tube with a glass funnel at the open end. Only one inhalation of [Pg 359] the mixture is used to determine the intensity of the odor. The position of any strength of odor on the scale depends upon the sensitiveness and judgment of the operator, but with one person conducting the entire test, the results have been found quite satisfactory. (See tables on pages 360 and 361.)
Substances which seem useful for producing skin burns are studied both on animals and on man. Dichloroethyl sulfide (mustard gas) is used as a basis of comparison. Several methods are available.
Direct Application. This method consists of the direct application of the compound itself to the skin, using a definite quantity (0.005 cc. or 0.005 mg.) over a definite area (5 square centimeters) of the skin. With such a quantity of mustard gas a rather severe burn on animals is produced. No precautions are taken to prevent evaporation from the skin since it is believed that in this way the test will approximate fairly closely the field conditions.
Vapor Tests. Preliminary tests with vapors of volatile compounds are best made by placing a small amount of the material on a plug of cotton in the bottom of a test tube enclosed in a larger test tube which acts as an air jacket. After about an hour at room temperature the mouth of the test tube is applied to the skin. The concentration is not known, but one is dealing practically with saturated vapor. If an exposure of from 30 to 60 minutes produces no effect, one is safe to assume that the compound is not sufficiently active to be of value as a skin irritant.
If quantitative results are desired, the apparatus shown in Fig. 119 is used. Dry air is blown through the bubbler, which is connected with a series of glass skin applicators. The concentration is determined in the usual way. The skin applicator consists of a small cylinder about 1.5 to 2 cm. in diameter and about 4 cm. long with a small glass handle attached on top. The opening is 1 cm. in diameter. When the concentration of the gas is constant, the exposure to the skin is made directly for any desired length of time. The skin irritant efficiency is judged by comparing the per cent of positive responses to approximately equal concentrations of the vapors, using mustard gas as a standard. [Pg 360]
TABLE I—Physical and Physiological Properties
of Chemicals Used as Stenches
| Chemical | Boiling Point, °C. |
Freezing Point, °C. |
Character of Odor |
Physiological Properties of Vapor |
Remarks |
|---|---|---|---|---|---|
| Amyl acetate | 148 | -75 (thick) |
Banana oil | Harmless | Pleasant to most people; disagreeable to some |
| Ethyl acetate | 77.4 | -83.8 | Fruity, pleasant | Harmless | |
| Amyl alcohol | 137.8 | Alcoholic | Harmless | ||
| Butyric acid | 162.3 | -7.9 | Very disagreeable | Harmless | |
| Valeric acid | 186.4 | -58.5 | Very disagreeable | Harmless | |
| Ethyl ether | 35 | -112.6 | Pungent | Soporific | |
| Phenyl isocyanide | 165 | Very disagreeable | Unknown | ||
| Allyl isothiocyanate | 151 | Mustard oil, disagreeable | Lachrymatory and toxic | ||
| Methyl isothiocyanate | 119 | 34 | Mustard oil, disagreeable | Lachrymatory and toxic | |
| Amyl isovalerate | 190 | Very disagreeable | Harmless | ||
| Butyl mercaptan | 97 | Very disagreeable | Harmless | ||
| Isobutyl mercaptan | 88 | Very disagreeable | Unknown | Probably harmless | |
| Ethyl mercaptan | 37 | -144.4 | Very disagreeable | Harmless | |
| Propyl mercaptan | 67 | Very disagreeable | Unknown | Probably harmless | |
| Methyl salicylate | 222.2 | -8.3 | Oil of wintergreen, pleasant | Harmless | |
| Amyl thioether | 95-98 | Very disagreeable | Unknown | Probably harmless | |
| Ethyl thioether | 92 | -99.5 | Very disagreeable | Unknown | Probably harmless |
| Carbon tetrachloride | 76.74 | -19.5 | Sweet, unpleasant | Harmless | |
| Chloroform | 62 | -63.2 | Sweet, agreeable | Soporific | |
| Iodoform | Decomposes | 119 | Unpleasant | Harmless | |
| Artificial musk | Pleasant | Harmless | Unpleasant in higher concentration |
||
| Nitrobenzene | 209.4 | 5.71 | Almonds, pleasant | Toxic | |
| Oil of peppermint | Pleasant | Harmless | |||
| Pyridine | 115.2 | -42 | Very disagreeable | Toxic |
[Pg 361]
TABLE II—Results of Measurement
of the Intensity
of Various Stenches
| Chemical | Volumes of the Chemical, as a Perfect Gas, per Million Volumes of Air, Intensity of Odor |
||||
|---|---|---|---|---|---|
| Detectable | Faint | Quite Noticeable |
Strong | Very Strong |
|
| Amyl acetate | 7 | 10 | 13 | 90 | 246 |
| Ethyl acetate | 190 | 339 | 615 | 1236 | 1753 |
| Amyl alcohol | 63 | 83 | 123 | 439 | 601 |
| Butyric acid | 2.4 | 6 | 18 | 91 | 161 |
| Valeric acid | 7 | 29 | 125 | 332 | 962 |
| Ethyl ether | 1923 | 3352 | 4927 | 5825 | 19982 |
| Butyl mercaptan | 6 | 12 | 18 | 38 | 56 |
| Isobutyl mercaptan | 3.5 | 5 | 7 | 11 | 16 |
| Ethyl mercaptan | 18 | 35 | 73 | 141 | 198 |
| Propyl mercaptan | 2 | 7 | 9 | 14 | 17 |
| Amyl thioether | 0.2 | 1 | 1.6 | 1.7 | 2.2 |
| Ethyl thioether | 3 | 12 | 29 | 61 | 74 |
| Allyl isothiocyanate | ?2 | 3 | 6 | 8 | 50 |
| Methyl isothiocyanate | 5 | 13 | 23 | 36 | 48 |
| Amyl isovalerate | 1.7 | 3 | 6 | 10 | 12 |
| Carbon tetrachloride | 718 | 1461 | 1588 | 4964 | 6091 |
| Chloroform | 674 | 1389 | 2600 | 5887 | 19528 |
| Iodoform | 1.1[35] | ||||
| Artificial musk | |||||
| Nitrobenzene | 29 | 36 | 44 | 114 | 296 |
| Phenyl isocyanide | 0.5 | 1 | 3 | 10 | 25 |
| Pyridine | 10 | 45 | 93 | 700 | 1764 |
| Methyl salicylate | 16.1 | 23 | 29 | 244[36] | |
| Oil of peppermint | |||||
| Chemical | Milligrams
of Chemical per Cu. Ft. of Air, Intensity of Odor |
||||
|---|---|---|---|---|---|
| Detectable | Faint | Quite Noticeable |
Strong | Very Strong |
|
| Amyl acetate | 1.1 | 1.5 | 2 | 14 | 38 |
| Ethyl acetate | 19.4 | 34.6 | 63 | 126 | 191 |
| Amyl alcohol | 6.4 | 8.5 | 13 | 45 | 61 |
| Butyric acid | 0.3 | 0.6 | 2 | 9 | 16 |
| Valeric acid | 0.8 | 3.4 | 15 | 39 | 114 |
| Ethyl ether | 165.1 | 287.7 | 423 | 500 | 1715 |
| Butyl mercaptan | 0.5 | 1.0 | 2 | 3 | 5 |
| Isobutyl mercaptan | 0.2 | 0.5 | 0.7 | 1 | 2 |
| Ethyl mercaptan | 1.3 | 2.5 | 5 | 10 | 14 |
| Propyl mercaptan | 0.2 | 0.6 | 0.8 | 1.2 | 1.6 |
| Amyl thioether | 0.04 | 0.2 | 0.3 | 0.4 | 0.5 |
| Ethyl thioether | 0.3 | 1.2 | 3 | 6 | 8 |
| Allyl isothiocyanate | 0.2 | 0.3 | 0.7 | 0.9 | 6 |
| Methyl isothiocyanate | 0.4 | 1.1 | 2 | 3 | 4 |
| Amyl isovalerate | 0.4 | 0.5 | 1 | 2 | 2.3 |
| Carbon tetrachloride | 128 | 260 | 283 | 886 | 1087 |
| Chloroform | 93 | 192 | 360 | 816 | 1321 |
| Iodoform | 0.5[37] | ||||
| Artificial musk | 0.001[38] | ||||
| Nitrobenzene | 4 | 5 | 6 | 16 | 42 |
| Phenyl isocyanide | 0.06 | 0.1 | 0.4 | 1 | 3 |
| Pyridine | 0.9 | 4 | 9 | 64 | 162 |
| Methyl salicylate | 2.8 | 4 | 5 | 43[39] | |
| Oil of peppermint | 0.68 | 0.9 | 3 | 9.5 | 9.9 |
| Chemical | Milligrams
of Chemical per Liter of Air, Intensity of Odor |
||||
|---|---|---|---|---|---|
| Detectable | Faint | Quite Noticeable |
Strong | Very Strong |
|
| Amyl acetate | 0.039 | 0.053 | 0.067 | 0.478 | 1.326 |
| Ethyl acetate | 0.686 | 1.224 | 2.219 | 4.457 | 6.733 |
| Amyl alcohol | 0.225 | 0.300 | 0.442 | 1.581 | 2.167 |
| Butyric acid | 0.009 | 0.021 | 0.066 | 0.329 | 0.580 |
| Valeric acid | 0.029 | 0.119 | 0.523 | 1.394 | 4.036 |
| Ethyl ether | 5.833 | 10.167 | 14.944 | 17.6667 | 60.600 |
| Butyl mercaptan | 0.018 | 0.037 | 0.055 | 0.120 | 0.177 |
| Isobutyl mercaptan | 0.008 | 0.018 | 0.025 | 0.041 | 0.060 |
| Ethyl mercaptan | 0.046 | 0.088 | 0.186 | 0.357 | 0.501 |
| Propyl mercaptan | 0.006 | 0.020 | 0.028 | 0.043 | 0.054 |
| Amyl thioether | 0.001 | 0.007 | 0.0115 | 0.012 | 0.015 |
| Ethyl thioether | 0.012 | 0.042 | 0.107 | 0.223 | 0.271 |
| Allyl isothiocyanate | 0.008 | 0.012 | 0.024 | 0.030 | 0.201 |
| Methyl isothiocyanate | 0.015 | 0.039 | 0.067 | 0.108 | 0.144 |
| Amyl isovalerate | 0.012 | 0.018 | 0.039 | 0.072 | 0.082 |
| Carbon tetrachloride | 4.533 | 9.222 | 10.024 | 31.333 | 38.444 |
| Chloroform | 3.300 | 6.800 | 12.733 | 28.833 | 46.666 |
| Iodoform | 0.018[40] | ||||
| Artificial musk | 0.00004[41] | ||||
| Nitrobenzene | 0.146 | 0.178 | 0.222 | 0.563 | 1.493 |
| Phenyl isocyanide | 0.002 | 0.005 | 0.013 | 0.042 | 0.105 |
| Pyridine | 0.032 | 0.146 | 0.301 | 2.265 | 5.710 |
| Methyl salicylate | 0.100 | 0.145 | 0.179 | 1.526[42] | |
| Oil of peppermint | 0.024 | 0.032 | 0.109 | 0.332 | 0.348 |
[Pg 362] Touch Method. This method consists of dipping a small glass rod drawn to a needle-like end to the depth of 1 mm. in the compound and then quickly touching the skin. The method is qualitative only.
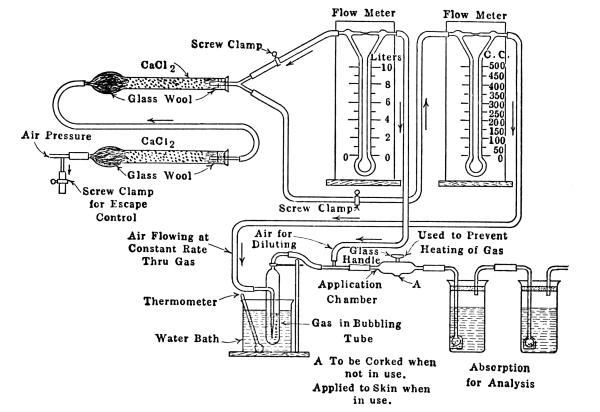
Fig. 119.—Skin Irritant Vapor Apparatus.
Use of Solutions. Alcohol, kerosene, olive oil, carbon tetrachloride and other solvents may be used for the purpose of determining the lowest effective concentration of a substance, and for the determination of the relative skin irritant efficiencies of various compounds. Since the skin irritants were scarcely ever used in this form in the field, that is, in solution, the method is not as satisfactory as the vapor method.
[Pg 363]
Fundamentals of War. The underlying fundamental principles of Chemical Warfare are the same as for all other arms. Because of this, it is worth while, and even necessary, to understand the applications of Chemical Warfare, for us to go back and study the work of the masters in war from the dawn of history down to the present. When we do that we find that the underlying fundamental principles of war remain unchanged. They are the same today as they were in the time of Demosthenes, and as they will be 10,000 years from now. It is an axiom that the basis of success in war is the ability to have at the decisive point at the decisive moment a more effective force than that of the enemy. This involves men and materials. It involves courage, fighting ability, and the discrimination and energy of the opposing commanders.
Another fundamental is that no success is achieved without positive action; passive resistance never wins. These are really unchanging fundamentals. We may also say that the vigor of attack, the speed of movement of men and supplies, and the thorough training of men in the use of the weapons of war are unchanging requirements, but outside of these everything is subject to the universal law of change.
Grecian Phalanx and Roman Legion. The last word in the development of human strength as a battle weapon was illustrated by the Grecian phalanx with its sixteen rows of men, the spears of each row being so adjusted that all reached to the front line. That phalanx [Pg 364] could not be stopped by any other human formation that met it face to face. To overcome it required a Roman legion that could open up and take the phalanx in the flank and rear. In the same way, the elephants of the Africans and the chariots of the Romans with their great swords swept all in front of them, until the Roman Legion, opening up into smaller groups allowed the elephants and chariots to pass through only to close in on them from the rear. Then and then only did those engines of war disappear forever.
Frederick the Great. Frederick the Great, realizing that rapidity of fire would win on the fields of battle where he fought, trained his men to a precision of movement in close order probably never achieved by any other troops in the world and then added to their efficiency by teaching them to load and fire muskets at double the rate of that of his adversaries. He was thus enabled to concentrate at the decisive points a preponderance of power, which swept all his enemies before him.
Napoleon. Napoleon achieved the same decisive power in a different way. Realizing that his French troops could not stand the rigorous training that the Prussians underwent, he trained them to fight with great enthusiasm, to travel long distances with unheard of swiftness, and to strike the enemy where least expected. He added to that a concentration of artillery until then not thought of as possible on the field of battle. He, of course, had also a genius for organizing and keeping up his supply.
Grant and Jackson. Grant at Vicksburg and Stonewall Jackson in the Shenandoah Valley and at Chancellorsville, achieved the same results in different ways. In every case the fundamental principle of concentrating the greatest force at the decisive point at the vital moment in the battle remained the same. The methods for achieving that end change with every age, and every commander of world-wide renown developed something new or used an old method in a new way. And that is the fundamental requirement for a successful general. Hannibal, Hasdrubal, Cæsar, Napoleon, Frederick the Great, Scott, Grant, and Jackson were all independent thinkers. Each and every one dared to do something that every other general and statesman of his time told him [Pg 365] could not be done or that would bring about disaster. They had the courage of their convictions. They had the courage to think out new ideas and to develop them, and then they had the courage to carry through those convictions, not alone against the opposition of the enemy, but against the opposition of their own people, both in the field and at home. And we may be perfectly sure that in each case had these men not done the things they did, they would have gone down to oblivion just as has been the case with millions of others who tried the usual methods in the usual way.
Chemical Warfare Latest Development. Chemical Warfare is the latest development of war. So far as the United States is concerned, it is considerably less than four years old. It is the most scientific of all methods of fighting and also the most universally applicable to all other methods of making war. The use of poisonous and irritating gases in war is just as fundamental as the introduction of gunpowder. In fact, they have an even wider application to war than powder itself.
Necessity for New Methods. The idea that has been expressed above is that the General Staff and the Army commander who sticks to old and tried methods and who is unwilling to try with all his might new developments, will never achieve any first class success. The General Staffs and the generals of the future that win wars will be the ones who make the most vigorous and efficient use of Chemical Warfare materials. They cannot confine this use to the artillery, to Aviation, to Special Gas Troops, or to any other single branch of the war machine. They must make use of it in every way.
What Is Meant by Gas. It must be understood that by gases we refer to materials that injure by being carried to the victim in the air. The word “gas” has nothing whatever to do with the condition of the material when in the shell, or the bombs, or the cylinders before released. In every case, the gases are liquids or solids. When the containers are broken open the liquids are volatilized either by the gas pressure or by the force of the explosion of the bomb.
Groups of Gases. Chemical Warfare gases are divided into three great groups. So far as their actual tactical use on the field of [Pg 366] battle is concerned, there are only two groups—persistent and non-persistent. The third is the irritant group. This group affects the eyes and the lungs so as to make the victim very uncomfortable if not completely incapable of action in quantities so small as to cause no injury that lasts more than a few hours. The quantities of such gases needed to force the wearing of the mask is ¹/₁₀₀₀ that needed to cause the same discomfort by the really poisonous gases, such as phosgene. They, therefore, have a very great economic value in harassing the enemy by forcing him to wear masks and to take other precautions against gas. And no matter how perfect gas masks and gas-proof clothing become, their long-continued use will cut down physical vigor in an ever increasing ratio until in two or three days an army may be totally incapacitated.
Smoke. In Chemical Warfare materials we have another great group which will probably be equal in the future to the three groups just mentioned. That is common smoke. Smoke has a variety of uses. By the simple term “smoke” is meant smokes that are not poisonous or irritating. Such smokes offer a perfect screen against enemy vision, whether it is the man who sights the machine gun, the observer in the lookout station, the cannoneer or even the aeroplane observer. Every shot through impenetrable smoke is a shot in the dark and has a tenth or even less chance of hitting its mark. Smoke affords a means of decreasing the accuracy of firing, much the same as night decreases it, without the inherent difficulties of night action.
Peace Strategy. The strategy of successful war involves the strategy of peace. This has been true from the days when David with his sling-shot slew Goliath, down to the present moment. We don’t always think of it in connection with war, but back of every successful war has been preparation during peace. It may have been incidental preparation such as the training of men in fighting Indians, and in creating public sentiment favorable to an independent nation that preceded the Revolutionary War. It may, on the other hand, have been a deeply studied policy such as that of the Germans prior to the World War. They tried and generally quite successfully, to coördinate all peace activities toward the day when a war should come that would [Pg 367] decide the future destiny of the German Empire, and it was only because of that study in peace that Germany almost single-handed was able to stand out for more than four years against the world. The Allies came near losing that war because they did not appreciate that the strategy of efficient war had to be preceded by the strategy of peace.
Chemical Warfare an Example. Chemical warfare is a particularly good example of this fact. Prior to the World War we had acknowledged, and without any misgivings, that Germany led the world in chemistry, that it produced most of the dyes in the world, and to a large extent the medicines of the world. We felt that when American needs showed it to be advisable we could take up chemistry and chemical production and soon excel the Germans. We had not reckoned on the suddenness of war.
We were just getting ready with chemicals, and that included powders and high explosives, when the war closed. And yet we had had not only eighteen months’ intensive preparation after our own entry into the World War, but also the preparation of great steel institutions and powder factories for nearly three years in manufacturing supplies for the Allies who preceded us in the war.
Coal Tar. The World War opened the eyes of England, France and Japan as well as the United States. Each of them today is struggling to build up a great chemical industry as the very foundation of successful war. Few of us realized prior to the World War that in the black, sticky mess called coal tar from the coking of coal or the manufacture of gas from coal and oil, was stored up most of the high explosives used in war, the majority of the poison gases, a great deal of the medicines of the world, and nearly all the dyes of the world. The Germans realized it and in their control over methods of using this material, together with the great commercial plants developed to manufacture it, as well as with the trained personnel that must go with such plants, were enabled, when blockaded on land and sea, to furnish the munitions, the clothing and the food needed for four and one-half years of war.
Great Chemical Industries. Thus it is that our Government today [Pg 368] is giving most serious heed to the need of building up a great chemical industry in the United States. We have the raw materials. We need only the factories and the trained men that go with them. We need, of course, in addition to the development of the coal tar industry, a production of heavy chemicals such as chlorine, sulfuric acid and the like, all of which, however, are bound together by community interest in peace as well as in war.
Reserves of Chemists. A part of the strategy of peace is the card-indexing of the manpower of a nation divided into special groups. In one great group must come those who have a knowledge of chemistry and the chemical industries. That must be so worked out that if war should come on a moment’s notice, within twenty-four hours thereafter every chemist could be given his job, jobs extending from the firing line to the research laboratory. And that is the task of the Chemical Warfare Service. And right here it is well to know that Congress, among the other features of its Army Reorganization Act of June 4, 1920, provided for a separate Chemical Warfare Service with these powers:
Chemical Warfare Powers
The Chief of the Chemical Warfare Service under the authority of the Secretary of War shall be charged with the investigation, development, manufacture, or procurement and supply to the Army of all smoke and incendiary materials, all toxic gases, and all gas defense appliances; the research, design, and experimentation connected with chemical warfare and its material; and chemical projectile filling plants and proving grounds; the supervision of the training of the Army in chemical warfare, both offensive and defensive, including the necessary schools of instruction; the organization, equipment, training, and operation of special gas troops, and such other duties as the President may from time to time prescribe.
Why Power Is Needed. These rather broad powers indicate that Congress realized the unity of effort that must be made from the research laboratory to the firing line if America was to keep pace with Germany or any other nation in chemical warfare. Some have raised the question as to whether a service should be both supply and combat. [Pg 369] Perhaps the best answer to that question is that so organized Chemical Warfare was a success in the World War. It was a success notwithstanding it had to be developed in the field six months after our entry into the war and with no precedents, no materials, no literature and no personnel. Through its officers on the staffs of commanding generals of armies, corps and divisions, and through its fighting gas troops in the front line, it was enabled to direct its research, development and manufacture more quickly along lines shown to be necessary by every change in battle conditions, than any other service.
Chemical Warfare Troops. And why should there not be fighting Chemical Warfare troops? They fight under exactly the same orders as all other troops. They conform to the same general plan of battle. They bring, however, to that battle experts in a line that it takes a long time to master. And where has there been any live commander in the world’s history who refused aid from any class of troops that might help him win?
Specialists in War. The wars of the future will become more and more wars of the specialists. Your Infantry may remain the backbone of the fighting force, but if it has not the Artillery, the Aviation, the Chemical Warfare, the Engineers, the tanks and other specialists to back it up, it will be overcome by the army which has such specialists. Indeed the specialist goes into the very organization of the Infantry itself with its machine gun battalions, its tank battalions, and as now proposed, the Infantry light howitzer companies.
Duties of Chemical Warfare Staff Officers. The Chemical Warfare officers on the staff of armies, corps and divisions are there for the purpose of giving expert advice as to the quantities of chemical materials available, the best conditions for using them, and the best way of avoiding the effects of enemy gas upon our own troops. The conditions that must be kept in mind are so many that no other officer can be expected to master and keep them if he does his own work well. The general staff officers and commanding generals will not have the time to even try to remember the actual effects of clouds, wind, rain, trees, valleys, villages and plains upon each and every gas. They must depend upon the Chemical Warfare officer for accurate information along [Pg 370] those lines, and if he cannot furnish it they will have to secure some one who can. The history of war is filled with the names of generals who failed because they could not forget how to command a company. These Chemical Warfare officers will also furnish all data as to supply of chemical warfare materials, and will furnish the best information along lines of training, whether for defensive or offensive use of gas.
Gas Used by all Arms. As before stated, we cannot confine the use of gas to any one arm. We may then ask why, if it is applicable to all arms, it should need special gas troops. Special gas troops are for the purpose of putting off great quantities of chemical warfare materials by special methods that are not applicable to any other branch now organized or that any other branch has the time to master. Long-range firing of gas by the artillery can be done just as well by the artillery as by gas troops. Why? Because in the mechanics of firing chemical ammunition there is no difference whatever from the mechanics of firing high explosives or shrapnel. The same will be true of gas rifle grenades and smoke candles in use by the Infantry. The same will be true of the dropping of gas bombs and the sprinkling of gas by the aeroplanes. In this connection just remember that all of the army is trained in first aid, but in addition we have our ambulance companies, our hospitals, and our trained medical personnel.
Arguments Against Use of Gas. It has been many times suggested since the Armistice that the use of poisonous gas in war may be done away with by agreement among nations. The arguments against the use of gas are that it is inhumane and that it might be used against non-combatants, especially women and children. The inhumanity of it is absolutely disproven by the results of its use in the World War. The death rate from gas alone was less than one-twelfth that from bullets, high explosives and other methods of warfare. The disability rate for gas patients discharged was only about one-fourth that for the wounded discharged for other causes. The permanently injured is likewise apparently very much less than from other causes.
Humanity. No reliable statistics that we can get show that gas [Pg 371] in any way causes tuberculosis any more than a severe attack of bronchitis or pneumonia causes tuberculosis. Since its principal effects are upon the lungs and, therefore, hidden from sight, every impostor is beginning to claim gassing as the reason for his wanting War Risk benefits from the Government. We do not claim there may not be some who are suffering permanent injuries from gas, and we are trying very hard to find out from the manufacturers of poisonous gases and allied chemicals if they have any authentic records of such cases. So far the results indicate that permanent after-effects are very rare.
As to non-combatants, certainly we do not contemplate using poisonous gas against them, no more at least than we propose to use high explosives in long-range guns or aeroplanes against them. The use of the one against non-combatants is just as damnable as the other and it is just as easy to refrain from using one as the other.
Gas Cannot be Abolished. As to the abandonment of poison gas, it must be remembered that no powerful weapon of war has ever been abandoned once it proved its power unless a more powerful weapon was discovered. Poisonous gas in the World War proved to be one of the most powerful of all weapons of war. For that reason alone it will never be abandoned. It cannot be stopped by agreement, because if you can stop the use of any one powerful weapon of war by agreement you can stop all war by agreement. To prepare to use it only in case it is used against you is on the same plane as an order that was once upon a time issued to troops in the Philippine Islands. That order stated in substance that no officer or soldier should shoot a savage Moro, even were he approaching the said officer or soldier with drawn kriss (sword), unless actually first struck by such savage. Every officer preferred, if necessary, to face a court-martial for disobedience of such an order rather than allow a savage Moro with a drawn kriss to get anywhere near, let alone wait until actually struck.
Let the world know that we propose to use gas against all troops that may be engaged against us, and that we propose to use it to the fullest extent of our ability. We believe that such a proposition will do more [Pg 372] to head off war than all the peace propaganda since time began. It has been said that we should not use gas against those not equipped with gas. Then why did we use repeating rifles and machine guns against Negritos and Moros armed only with bows and arrows or poor muskets and knives. Let us apply the same common sense to the use of gas that we apply to all other weapons of war.
Effect on World War Tactics. A very brief study of the effects of chemical warfare materials on the strategy of the World War will indicate its future. It began with clouds of chlorine let loose from heavy cylinders buried under the firing trench. These took a long time to install and then a wait, sometimes long, sometimes brief, for a favorable wind, but even at that these cloud gas attacks created a new method of fighting and forced new methods of protection. Gas at once added a tremendous burden to supply in the field, to manufacture, and to transportation, and in a short time even made some decided changes in the tactics of the battle field itself.
Cloud Gas. The fact that the gas cloud looked like smoke is responsible for the name “cloud gas.” Really all gases are nearly or wholly invisible, but those which volatilize suddenly from the liquid state so cool the air as to cause clouds of condensed water vapor. The cloud obscured everything behind and in front of it. It led the German to put off fake smoke clouds and attack through them, thus taking the British at a tremendous disadvantage. Then and there began a realization of the value of smoke. Cloud gas was also the real cause of the highly organized raid that became common in every army during the World War. The real purpose in the first raids, carried out by means of the box barrage, was to find out whether or not gas cylinders were being installed in trenches.
These raids finally became responsible, in a large measure, for driving the old cloud gas off the field of battle. It did not, however, stop the British from putting off cloud gas attacks in 1918 by installing their gas cylinders on their light railway cars and then letting the gas loose from the cylinders while still on the cars. This enabled them to move their materials to the front and put off gas attacks on a few hours’ notice when the wind was right. [Pg 373]
Toxic Smoke Candles. To-day we have poisonous smokes that exist in solid form and that are perfectly safe to handle until a fuse is lighted. The so-called candles will be light enough so that one man can carry them. With these, cloud gas can be put off on an hour’s notice when wind and weather conditions are right, no matter how fast the army may be moving and whether on the advance or in retreat. Cloud gas will usually be put off at night because the cloud cannot be seen, because then men are tired and sleepy, and all but the most highly trained become panicky. Under those conditions the greatest casualties result. The steadiness of wind currents also aids cloud gas attacks at night.
Value of Training in Peace. And this brings up the value of training in peace. We are frequently asked, “Why do you need training with masks in peace; why do you need training with actual gas in peace; cannot these things be taught on short notice in war?” The answer is, “No!” Nothing will take the place of training in peace.
All of us recall that early in the war the Germans spread broadcast charges that the Allies were using unfair and inhumane methods of fighting because they brought the Ghurka with his terrible knife from Asia and the Moroccan from Africa. And we all know that after a time the Germans ceased saying anything about these troops. What was the cause? They were not efficient. Just as the Negro will follow a white officer over the top in daylight and fight with as much energy and courage and many times as much efficiency as the white man, he cannot stand the terrors of the night, and the same was true of the Ghurka and the Moroccan.
All the Allies soon recognized that fact as shown by their drawing those troops almost entirely away from the fighting lines. In some cases dark-skinned troops were kept only as shock troops to be replaced by the more highly developed Caucasian when the line had to be held for days under the deadly fire of the counter attack. The German idea, and our own idea prior to the World War, was that semi-savages could stand the rigors and terrors of war better than the highly sensitive white man. War proved that to be utterly false.
Familiarity with Gas Necessary. The same training that makes for [Pg 374] advancement in science, and success in manufacture in peace, gives the control of the body that holds the white man to the firing line no matter what its terrors. A great deal of this comes because the white man has had trained out of him nearly all superstition. He has had drilled into him for hundreds of years that powder and high explosive can do certain things and no more. If the soldier is not to be afraid of gas we must give him an equal knowledge of it, its dangers, and its limitations. The old adage says, “Familiarity breeds contempt.” Perhaps that is not quite true, but we all know that it breeds callousness and forgetfulness; that the man manufacturing dynamite or other more dangerous explosives takes chances that we who do not engage in such manufacture shudder at.
Edgewood Chemists Not Afraid. All of this has direct application to training with chemical warfare materials in peace. We believe that all opposition to chemical warfare today can be divided into two classes—those who do not understand it and those who are afraid of it—ignorance and cowardice. Our chemists at Edgewood Arsenal are every day toying with the most powerful chemical compounds; toying with mixtures they know nothing of, not knowing what instant they may induce an explosion of some fearful poisonous gas. But they have learned how to protect themselves. They have learned that if they stop breathing and get out of that place and on the windward side they are safe. They have been at that work long enough to do that automatically.
Staff Officers Must Think of Gas in Every Problem. The staff officer must train the army man in peace with all chemical warfare materials or he will lose his head in war and become a casualty. The general staff officers and commanding generals must so familiarize themselves with these gases and their general use that they will think them in all their problems just exactly as they think of the Infantry, or of the Cavalry, or of the tanks or of the Artillery in every problem. On them rests the responsibility that these gases are used properly in battle. If plans before the battle do not include these materials for every arm and in the proper quantities of the proper kinds they will not be used properly on the field of battle and on them will rest the responsibility. [Pg 375]
They are not expected to know all the details of gases and their uses, but they will be expected to consider the use of gas in every phase of preparing plans and orders and then to appeal to the chemical warfare officers for the details that will enable them to use the proper gases and the proper quantities. They cannot go into those details any more than they can go into the details of each company of infantry. If they try to do that they are a failure as staff officers.
Effect of Masks on Troops. The very best of masks cause a little decrease in vision, a little increase in breathing resistance, and a little added discomfort in warm weather, and hence the soldier must learn to use them under all conditions. But above all in the future he must be so accustomed to the use of the mask that he will put it on automatically—almost in his sleep as it were. We have tear gases, today, so powerful and so sudden in their action that it is doubtful if one man out of five who has had only a little training can get his mask on if subject to the tear gas alone—that is, with tear gas striking him with full force before he is aware of it.
Effectiveness of Gas in World War. In the past war more than 27 out of every 100 Americans killed and wounded suffered from gas alone. You may say that many of the wounds were light. That is true; but those men were put out of the battle line for from one to four months—divisions, corps and armies almost broken up—and yet the use of gas in that war was a child’s game compared to what it will be in the future.
It is even said that many of them were malingerers. Perhaps they were, but do you not suppose that there were at least as many malingerers among the enemy as there were in our own ranks? Furthermore, if you can induce malingering it is a proper method of waging war, and unless our boasted ability is all a myth we should have fewer malingerers under conditions of battle than any other nation.
Strategy of Gas at Picardy Plains. Let us go back now to the strategy of gas in war. Following the cloud gas came tear gases and poisonous gases in shells and bombs. A little advance in tactics here and a little there, the idea, though, in the early days being only to produce casualties. As usual the Germans awoke first to the fact that [Pg 376] gas might be used strategically and on a large scale. And thus we find that ten days before he began the battle of Picardy Plains he deluged many sections of the front with mustard gas. He secured casualties by the thousands, but he secured something of greater importance. He wore out the physical vigor and lowered the morale of division after division, thus paving the way for the break in the British Army which almost let him through to the sea.
He used non-persistent gases up to the very moment when his own men reached the British lines, thereby reducing the efficiency of British rifle and artillery fire and saving his own men. And this is just a guide to the future. A recent writer in the Field Artillery Journal states that gas will probably not be used in the barrage because of its probable interference with the movement of our own troops. In making that statement he forgot the enemy and you cannot do that if you expect to win a war.
Gas in Barrages. In the future we must expect the enemy to be in a measure as well prepared in chemical warfare as we are. Let us consider the special case of our own men advancing to the attack behind a rolling barrage. We will consider also that the wind is blowing toward our own troops. Obviously under those conditions the wind will blow our own gas back onto our troops. Will we use gas in that barrage? We certainly will! Because with the wind blowing toward our own troops we have the exact ideal condition that the enemy wants for his use of gas. He will then be deluging our advancing troops with all the gas he can fire, in addition to high explosives and shrapnel. Our men must wear masks and take every precaution against enemy gas. How foolish it would be not to fire gas at the enemy under those conditions. If we did not fire gas we would leave him entirely free from wearing masks, and entirely free from taking every other precaution against gas while our own troops were subject to all the difficulties of gas. No, we will fire gas at him in just as great quantities as we consider efficient. And that is just a sample of what is coming on every field of battle—gas used on both sides by every method of putting it over that can be devised.
World War Lessons Only Guide Posts. Example of Book Worms. Every [Pg 377] lesson taught by the World War must be taken as a guide-post on the road to future success in war. No use of gas or other materials in the past war must be taken as an exact pattern for use in any battle of the future. Too much study, too much attention to the past, may cause that very thing to happen. A certain general commanding a brigade in the Argonne told me just recently that while the battle was going on a general staff officer called him on the telephone and asked him what the situation was. He gave it to him. The staff officer then asked, “What are you doing?” and he told him. The staff officer replied, “Why, the book doesn’t say to do it that way under such conditions.” There you have the absurd side of too much study and too close reliance on details of the past.
The battle field is a perfect kaleidoscope. The best we can hope to get out of books is a guide—something that we will keep in our minds to help us decide the best way to meet certain situations. He who tries to remember a particular position taught in his school with the idea of applying that to actual use in battle is laying the foundation for absolute failure. Your expert rifleman never thinks back when he goes to fire a shot as to just what his instructor told him or what the book said. He just concentrates his mind on the object to be attained, using so far as comes to him facts he has learned from books or teachers. Your general and your staff must do the same.
Infantry Use of Gas. A few words about how we will use gas in the future. We will start with the Infantry. The Infantry as such will use gas in only two or three ways. They will use some gas in rifle grenades, and a great deal more smoke. We speak of the rifle grenade because in our opinion the hand grenade is a thing of the past. We do not believe there will ever be used in the future any grenade that is not applicable to the rifle. The Infantry will probably often carry large quantities of gas in the shape of the toxic smoke candle. These materials being solids may be shot up by rifles or artillery fire, run over by trucks or tractors, or trampled and still be harmless. It is only when the fuses are lighted and the material driven off by heat that they are dangerous. In using these candles under these conditions [Pg 378] you must have sufficient chemical warfare officers and soldiers to get the necessary control indicated by the sun, wind, woods, fogs, ravines and the like.
Cavalry Use of Gas. Next consider the Cavalry. The Cavalry will use gas practically the same as the Infantry. The chemical warfare troops will accompany the Cavalry with Stokes’ mortars or other materials to fire gases into small enemy strongholds that may be encountered whether machine gun nests, mountain tops, woods or villages. They will do this either against savages or civilized people. Methods of making these materials mobile for that purpose are already well under way. If against savages and one does not want to kill them, use tear gases—no better method of searching out hidden snipers in mountain tops, among rocks, or villages, in ravines, or in forests was ever invented.
Use of Gas by Tanks. The tanks will employ gas in the same way as the Infantry with the possibility, however, that they may be used to carry large quantities of gas on caterpillar tractors where otherwise it would be difficult to move the gas. This is not a certainty, but is a situation promising enough to warrant further study.
Artillery Use of Gas. Your Artillery will fire gas and smoke in every caliber of gun. There is a tendency now to limit gas to certain guns and howitzers and to limit smoke to even a smaller number of guns. This is a mistake that we are going to recognize. A very careful study of the records of the war show that more casualties were produced several times over by a thousand gas shells than by a thousand high explosive or shrapnel. And that is because gas has an inherent permanence that no other weapon of war has.
Permanency of Gas. The bullet whistles through the air and does its work or misses. The high explosive shell bursts, hurling its fragments that in a few seconds settle to earth, and its work is done. The shrapnel acts in the same way, but when one turns loose a shell of gas it will kill and injure the same as the high explosive shell and in the same length of time and in addition for some minutes thereafter. Even with the non-persistent gases, it will continue on its way, causing death or injury to every unprotected animal, man or beast in its [Pg 379] path. With the persistent gases, the materials from each shell may persist for days.
Variety of Uses of Gas. This brings up the point of the great variety of uses to which gas can be put. The non-persistent gas may be used at all times where one wants to get rid of it in a few moments—the persistent gas wherever one wants to keep the enemy under gas for days at a time. We will use mustard gas on strong points in the advance, on flanks, on distant areas one will not expect to be reached, and as our own protection of masks and clothing increases toward perfection we will use it on the very fields you expect to cross. Why? Because we will be firing it at the enemy for days before hand and we will cause him trouble all those days while we ourselves will encounter it for a few hours at the most. So do not think that mustard gas is only going to be used in defense in the future.
Solid Mustard Gas and Long-Range Guns. We will come to use chemical warfare materials just as high explosives and bullets are used today, even though at times we do suffer an occasional loss from our own weapons. Our Artillery in long-range guns where we want destruction will fill each shell with say 15 per cent gas and 85 per cent high explosive. We have a solid mustard gas that may be so used. We have tremendously powerful tear gases and irritating gases that may be so used. Being solids they do not affect the ballistic qualities of the shell. And what an added danger will mustard gas from every shell bring against railroad centers, rest villages, cantonments, cross-roads and the like. The results will be too great for any force to overlook such use.
Tear Gases in Shrapnel. We will probably use tear gas in most, if not all, of our shrapnel. The general idea now is that we should not put tear gas in all shrapnel because under certain conditions it will be blown back and harass our own troops. But as was said before, we must remember that the enemy will be using gas at all times as well as ourselves, and hence if we limit ourselves in any line we give the enemy an advantage. This use of gas by the Artillery will extend to all classes of guns—seacoast, field, turret and what not. [Pg 380]
Use of Gas by Air Service. Bombs. Let us next consider the Air Service. We naturally think of dropping gas in bombs when we speak of the use of gas by the Air Service. Gas will so be used and it will be used in bombs of perhaps a thousand pounds or even a ton in weight, at least 50 per cent of which will be gas. Such gases, however, will be of the non-persistent type—phosgene or similar ones. They will be used against concentration camps and cross-roads, on troops on the road in columns; against railroad centers and rest areas; in other words, against groups of men or animals.
Sprinkling. But that is not even the beginning of the use of gas by aeroplanes. Mustard gas, which is one-third again as heavy as water, and which volatilizes far slower than water, may be sprinkled through a small opening such as a bung hole in a tank that simply lets liquid float out. The speed of the aeroplane will atomize it. In this way, gas can be sprinkled over whole areas that must be crossed in battle. The Lewisite, of which we have heard considerable, will be used. It is less persistent than the mustard gas, but like mustard gas it produces casualties by burning. Unlike mustard gas, however, the burns from a quantity equal to three drops will usually cause death. The material can be made up by hundreds, even thousands, of tons per month.
We are working on clothing that will keep it out just as we have been and are working on clothing that will protect against mustard gas. But these gases are so powerful that if any opening be left in the clothing the gas will get through, so that even if we get clothing that will protect, it must cover every inch of the skin from head to foot. Besides the mask must be worn at all times.
Consider the burden put on any army in the field that would have to continually wear such complete protection. What a strain on the mentality of the men! As before said, to endure it at all we must train our men to think of such conditions, to face them in peace, and in order to do so we must actually use gas. Just as in the World War the highly trained Caucasian outdistanced the savage in endurance, just so [Pg 381] will the most highly trained men in the future outdistance all others in endurance.
Navy. We now come to the consideration of the Navy. The Navy will use gas both in its guns and in smoke clouds, and in some form of candle that will float. The toxic smokes that in high enough concentrations will kill are extraordinarily irritating in minute quantities—so minute they cannot be seen or felt for a few moments. Every human being on a ship must breathe every minute just as every human being everywhere must breathe every minute or die. A gas that gets into the ventilating system of a ship will go all through it and the Navy realizes it.
The Navy is studying how to keep the gas out of their own ships, and how to get it into the enemy’s ships. The toxic smokes may be dropped from aeroplanes or turned loose from under water by submarines. In either case they will give off smokes over wide areas through which ships must pass. Any defects will let these toxic smokes in and will force every man to wear a mask. Aeroplane bombs will come raining down on the ship or alongside of it either with toxic smokes or other terrible gases. White phosphorus that burns and cannot be put out wet or dry will be rained on ships. Yes, chemical warfare materials will be used by the Navy.
Gas Against Landing Parties. The use of gas against landing parties or to aid landing parties has come up in many ways. Our studies to date indicate that gas is a greater advantage to the defense against landing parties than to the offense. Mustard gas and the like may be sprinkled from aeroplanes, and while it will not float long on the water, it will float long enough to smear any small boats attempting to land. It can be sprinkled over all the areas that landing parties must occupy. Mustard gas may be placed in bombs or drums around all areas that are apt to be used as landing places and exploded in the face of advancing troops.
Storing Reserve Gases in Peace. And a word here about how long gases may be stored. One of the statements made by opponents of chemical warfare was that gas is a purely war time project and could [Pg 382] not be stored up in peace. We have today at Edgewood Arsenal some 1,400 tons of poisonous gases not including chlorine. Those gases have been manufactured, practically every ounce of them, for three years, and are yet in almost perfect condition. Our chemists believe they can be kept in the future for ten years and perhaps longer. Our gas shells then will have the life almost of a modern battleship, while the cost of a million will be but a fraction of the cost of a battleship. What I have just said applies particularly to liquid gases such as phosgene, chlorpicrin, and mustard gas. We know that many of the solids may be kept for far longer periods.
Storing Gas Masks. Our masks, too, we believe can be kept for at least ten years. Experience to date indicates that rubber deteriorates mainly through the action of sunlight and moisture that cause oxidation or other change in the crystalline structure of cured rubber. Accordingly, we are putting up masks today in hermetically sealed boxes. It is thus evident that we can store a reserve of masks and gases in peace the same as other war materials.
Use of Gas by Gas Troops. Now we come to the use of gas by special gas troops. In the war, Gas Troops used 4-inch Stokes’ mortars and 8-inch Livens’ projectors and in a very short time would have used a new portable cylinder for setting off cloud gas, using liquid gases, such as phosgene. They will use these same weapons in future wars. All of these are short-range weapons, but since the Livens’ bomb or drum contains 50 per cent of its weight in gas while the artillery shell contains 10 per cent, they have an efficiency away beyond that of artillery or any other method of discharging gas except cloud gas. They will, therefore, produce more casualties than any other method known for the amount of material taken to the front. These short-range weapons were developed by the British for trench use and not for open warfare, and yet our troops developed methods with the Stokes’ mortars that enabled them to keep up with many of the Infantry divisions.
Phosphorus and Thermit Against Machine Gun Nests. The use of phosphorus and thermit against German machine gun nests by the Gas [Pg 383] Troops is well known. How effective it was is not known to so many. Phosphorus and thermit were so used from the early days of the Marne fight in the latter part of July, 1918, to the very close of the war. There is no recorded instance where the Gas Troops failed to silence machine gun nests once the machine guns were located. In the future Gas Troops will put off the majority of all cloud gas attacks even with toxic smoke candles.
Necessity for Training in Peace. This is an outline of the subject of chemical warfare. As stated in the beginning, the fundamental underlying principles for the successful use of poisonous gas is necessarily the same as for any other war materials. The necessity for continuous training in peace is just the same with chemical warfare as with the rifle, the machine gun, with field artillery or any other weapon of war. Indeed it is more so because the use of gas is so perfectly adaptable to night work. Men must be taught to take precautionary measures when so sleepy, tired and worn out that they will sleep through the roar of artillery.
How Chemical Warfare Should be Considered. We ask you only to look at the use of chemical warfare materials as you look at the use of the artillery, infantry, cavalry, tanks or aeroplanes. Measure its possible future use; not simply by its use in the World War, but by considering all possible developments of the future. Remember that its use was barely four years old when the war closed, while the machine gun, the latest type of infantry weapon, had been known for more than one-third of a century. Chemical warfare developments are in the infant stage. Even those on the inside of chemical warfare when the Armistice was signed can see today things that are certain to come that were undreamed of at that time. This is bound to be so with a new weapon.
To sum up, gas is a universal weapon, applicable to every arm and every sort of action. Since we can choose gases that are either liquid or solid, that are irritating only or highly poisonous, that are visible or invisible, that persist for days or that pass with the wind, we have a weapon applicable to every act of war and for that matter, to every act of peace. But we must plan its use, remembering there is no [Pg 384] middle ground in war, it is success or failure, life or death. Remember also that training outruns production in a great war, that 5,000,000 men can be raised and trained before they can be equipped unless we with proper foresight build up our essential industries, keep up our reserve of supplies, and above all, keep such perfect plans that we can turn all the wheels of peace into the wings of war on a moment’s notice.
[Pg 385]
Chemical Warfare includes all gas, smoke and incendiary materials and all defensive appliances, of which the mask is the principal item, used by the Army. Some of the items or materials in both offense and defense are used by the entire Army, while a few are used only by Chemical Warfare troops.
The term “gas” is now taken to include all materials that are carried to the enemy by the air, after their liberation from cylinders, bombs or shell. It is necessary that this broad use of the term “gas” be thoroughly understood, because some of these materials are solids, while all others are liquids, until liberated from the containers at the time of the attack. These containers may be special cylinders for cloud gas attacks, special bombs for Livens’ projectors and mortars, or artillery shell, and even aviation bombs. Some of the liquids which have a very low boiling point volatilize quickly upon exposure to air, and hence require only enough explosive to open the shell and allow the liquid to escape. Practically all solids have to be pulverized by a large amount of high explosive, or driven off as smoke by some heating mixture.
Chemical warfare, besides being the newest, is the most technical and most highly specialized Service under the War Department. There is no class of people in civil life, and no officers or men in the War [Pg 386] Department, who can take up chemical warfare successfully until they have received training in its use. This applies not only to the use of materials in attack, but to the use of materials for defense. Ten years from now perhaps this will not be true. It is certainly hoped that it will not be. By that time the entire Army should be pretty thoroughly trained in the general principles and many of the special features of chemical warfare. If not, chemical warfare cannot be used in the field with the efficiency and success with which it deserves to be used. Furthermore, it is believed that within ten years the knowledge of the gases used in chemical warfare will be so common through the development of the use of these same materials in civil life, that it will not be so difficult, as at the present date, to get civilians who are acquainted with Chemical Warfare Service materials.
Chemical warfare materials were used during the war by Chemical Warfare Service troops, by the Artillery and by the Infantry. In the future the Air Service and Navy will be added to the above list. Chemical warfare, even under the inelastic methods of the Germans, proved one of the most powerful means of offense with which the American troops had to contend. To realize its effectiveness we need only remember that more than 27 out of every 100 casualties on the field of battle were from gas alone. Unquestionably many of those who died on the battlefield from other causes suffered also from gas. No other single element of war, unless you call powder a basic element, accounted for so many casualties among the American troops. Indeed, it is believed that a greater number of casualties was not inflicted by any other arm of the Service, unless possibly the Infantry, and even in that case it would be necessary to account for all injured by bullets, the bayonet, machine guns and hand grenades. This is true, in spite of the fact that the German was so nearly completely out of gas when the Americans began their offensive at St. Mihiel and the Argonne, that practically no gas casualties occurred during the St. Mihiel offensive, and only a very few until after a week of the Argonne fighting. Furthermore, the Germans knew that an extensive use of mustard gas against the American [Pg 387] lines on the day the attack was made, and also on the line that marked the end of the first advance a few days later, would have produced tremendous casualties. Judging from the results achieved at other times by an extensive use of mustard gas, it is believed that had the German possessed this gas and used it as he had used it a few other times, American casualties in the Argonne would have been doubled. In fact, the advance might even have been entirely stopped, thus prolonging the war into the year 1919.
A few words right here about the humanity of gas are not out of place, notwithstanding the Army and the general public have now so completely indorsed chemical warfare that it is believed the argument of inhumanity has no weight whatever. There were three great reasons why chemical warfare was first widely advertised throughout the world as inhumane and horrible. These reasons may be summed up as follows:
In the first place, the original gas used at Ypres in 1915 was chlorine, and chlorine is one of a group of gases known as suffocants—gases that cause death generally by suffocating the patient through spasms of the epiglottis and throat. That is the most agonizing effect produced by any gas.
The second reason was unpreparedness. The English had no masks, no gas-proof dugouts, nor any of the other paraphernalia that was later employed to protect against poisonous gas. Consequently, the death rate in the first gas attack at Ypres was very high, probably 35 per cent. As a matter of fact, every man who was close to the front line died. The only ones who escaped were those on the edges of the cloud of gas or so far to the rear that the concentration had decreased below the deadly point.
The third great reason was simply propaganda. It was good war propaganda to impress upon everybody the fact that the German was capable of using any means that he could develop in order to win a victory. He had no respect for previous agreements or ideas concerning warfare. This propaganda kept up the morale and fighting spirit of the Allies, and was thoroughly justifiable upon that score, even when it led to wild exaggeration. [Pg 388]
The chlorine used in the first attack by the German is the least poisonous of the gases now used. Those later introduced, such as phosgene, mustard gas and diphenylchloroarsine are from five to ten times as effective.
The measure of humanity for any form of warfare is the percentage of deaths to the total number injured by the particular method of warfare under consideration.
American Gas Casualties. The official list of casualties in battle as compiled by the Surgeon General’s office covering all cases reported up to September 1, 1919, is 258,338. Of these 70,752, or 27.4 per cent, were gas casualties. Also of the above casualties 46,519 resulted in death, of whom about 1,400 only were due to gas. From these figures it is readily deduced that while 24.85 per cent of all casualties from bullets and high explosives resulted in death, only 2 per cent of those wounded by gas resulted in death. That is, a man wounded on the battle field with gas had twelve times as many chances of recovery as the man who was wounded with bullets and high explosives.
Before taking up in some detail the methods of projecting gas upon the enemy, it is very desirable to understand the fundamentals of chemical warfare, in so far as they pertain to poisonous gases. Following the first use of pure chlorine all the principal nations engaged in the war began investigations into a wide range of substances in the hope of finding others more poisonous, more easily produced, and more readily projected upon the enemy. These investigations led to the use of a large number of gases which seriously complicated manufacture, supply, and the actual use of the gases in the field. Gradually a more rational conception of chemical warfare led both the Allies and the enemy to restrict the numbers of gases to a comparative few, and still later to divide all gases into three groups. Thus the German divided his into three groups known as (1) Green Cross, the highly poisonous non-persistent gases, (2) Blue Cross, or diphenylchloroarsine, popularly known as sneezing gas, and (3) Yellow Cross, highly persistent gases, such as mustard gas. In the American Chemical Warfare Service we have finally divided all gases into two primary groups. [Pg 389] These groups are known as “Non-persistent” and “Persistent.” The “Non-persistent” gases are those quickly volatilizing upon exposure to the air, and hence those that are carried away at once by air currents, or that in a dead calm will be completely dissipated into the surrounding air in a few hours. If sufficient high explosive be used to pulverize solids, they may be used in the same way, and to a large extent certain highly persistent liquid gases may have their persistency greatly reduced by using a large amount of high explosive, which divides the liquid into a fine spray. The “Persistent” group constitutes those gases that are very slowly volatilized upon exposure to the atmosphere. The principal ones of these now used or proposed are mustard gas and bromobenzylcyanide. For purposes of economy, and hence efficiency, certain gases, both persistent and non-persistent, are placed in a third group known as the “Irritant Group.” These gases are effective in extremely low concentrations against the lungs and other air passages, or the eyes. Diphenylchloroarsine, and some other solids when divided into minute particles by high explosive or heat, irritate the nose, throat and lungs to such an extent in a concentration of one part in ten millions of air as to be unbearable in a few minutes. The tear gases are equally powerful in their effects on the eyes. The irritating gases are used to force the wearing of the mask, which in turn reduces the physical vigor and efficiency of the troops. This reduction in efficiency, even with the best masks, is probably 25 per cent for short periods, and much more if prolonged wearing of the mask is forced.
One pound of the irritant gases is equal to 500 to 1,000 pounds of other gases when forcing the wearing of the mask alone is desired. The great economy resulting from their use is thus apparent. Due to the rapid evaporation of the non-persistent gases they are used generally only in dense clouds, whether those clouds be produced from cylinders or from bombs. These gases are used only for producing immediate casualties, as the necessary amount of gas to force the enemy to constantly wear his mask by the use of non-persistent gases alone could not possibly be taken to the front. [Pg 390]
Mustard gas, which is highly persistent and also attacks the lungs, eyes and skin of the body, may and will be used to force the wearing of the mask. It has one disadvantage when it is desired to force immediately the wearing of the mask, and that is its delayed action and the fact that it acts so slowly, and is usually encountered in such slight concentrations that several hours’ exposure are necessary to produce a severe casualty. For these reasons the enemy may often take chances in the heat of battle with mustard gas, and while himself becoming a casualty, inflict quite heavy casualties upon opposing troops by continuing to operate his guns or rifles without masks. A powerful tear gas on the other hand forces the immediate wearing of the mask.
Chemical warfare troops, in making gas attacks, use cylinders for the cloud or wave attack, and the Livens’ projector and the 4-inch Stokes’ mortar for attacks with heavy concentrations of gas projected by bombs with ranges up to a mile. This distance will in the future probably be increased to 1½ or 1¾ miles. The original cylinders used in wave attacks were heavy, cumbersome and very laborious to install, and notwithstanding the wave attack was known to be the deadliest form of gas attack used in the war, fell into disrepute after the use of gas became general in artillery shells and in special bombs.
Cloud Gas. The Americans at once concluded that since cloud gas attacks were so effective, efforts should be made to make these attacks of frequent occurrence by decreasing the weight of the cylinders, and by increasing the portability and methods of discharging the cylinders. As early as March, 1918, specifications for cylinders weighing not more than 65 pounds, filled and completely equipped for firing, were cabled to the United States. They would have been used in large numbers in the campaign of 1919 had the enemy not quit when he did. Toxic smoke candles that are filled with solids driven off by heat will probably be the actual method in the future for putting off cloud attacks. The toxic smoke candle is perfectly safe under all conditions and can be [Pg 391] made in any size desired. Cloud gas attacks will be common in the future, and all plans of defense must be made accordingly. They will usually be made at night, when, due to fatigue and the natural sleepiness which comes at that time, men are careless, lose their way, or neglect their masks, and are thus caught unprepared. Experience in the war proved that a wave attack always produced casualties even, as several times occurred, when the enemy or the Allied troops knew some hours beforehand that the attack was coming. The English estimated these casualties to be 10 or 11 per cent of the troops exposed.
Livens’ Projectors. The second most effective weapon for using gas by gas troops was and will be the Livens’ projector. This projector is nothing less than the simplest form of mortar, consisting of a straight drawn steel tube and a steel base plate. As used during the World War by the Allies it did not even have a firing pin or other mechanism in the base, the electric wires for firing passing out through the muzzle and alongside the drum or projectile which was small enough to permit that method of firing. These were set by the hundreds, very close behind or even in front of the front line trenches. They were all fired at the same instant, or as nearly at the same instant as watches could be synchronized, and firing batteries operated. As discussed on 18 these mortars were emplaced deep enough in the ground to bring their muzzles practically level with the surface. It usually took several days to prepare the attack, and consequently allowed an opportunity for the enemy to detect the work by aeroplane photographs or by raids, and destroy the emplacements by artillery fire. It should be added, however, that notwithstanding this apparent great difficulty, very few attacks were broken up in that way. Nevertheless, in line with the general policy of the American troops to get away from anything that savored of trench warfare, and to make the fighting as nearly continuous as possible with every means available, the American Chemical Warfare Service set at work at once to develop an easy method of making projector attacks.
It was early found, that, if the excavation was made just deep enough so that the base plate could be set at the proper angle, the drums or projectiles were fired as accurately as when the projectors or mortars [Pg 392] were set so that the muzzles were level with the surface. The time required to emplace a given number of mortars in this way was only about one-fifth of that required for digging them completely in.
Coupled of course with these proposed improvements in methods, studies were being made and are still being made to produce lighter mortars, better powder charges, and better gas checks in order to develop the full force of the powder. Many improvements along this line can be made, all of which will result in greater mobility, more frequent attacks, and hence greater efficiency.
4-Inch Stokes’ Mortar. The Stokes’ mortar is not different from that used by the Infantry, except that it is 4-inch, while the Infantry Stokes’ is 3-inch. The 4-inch was chosen by the British for gas, as it was the largest caliber that could be fired rapidly and yet be moderately mobile. Its range of only about 1,100 yards handicapped it considerably. The poor design of the bomb was partly responsible for this. The powder charges also were neither well chosen nor well designed. It is believed that great improvements can be made in the shape of the bomb and in the powder charge, which will result in much longer range and high efficiency, while in no way increasing the weight of the bomb or decreasing the rate of fire. These last two weapons were used during the World War, and will be very extensively used in the future for firing high explosive, phosphorus, thermit and similar materials that non-technical troops might handle.
Since gas has proven without the shadow of a doubt, that it will produce more casualties for an equal amount of material transported to the front than any other substance yet devised, all troops using short range guns or mortars should be trained to fire gas whenever weather conditions are right. When weather conditions are not right, they should fire the other substances mentioned. The Livens’ projector with its 60 pound bomb, of which 30 pounds will be gas or high explosive, is a wonderful gun up to the limit of its range. The bomb, not being pointed, does not sink into the ground, and hence upon exploding exerts the full force of high explosive upon the surroundings, whether bombs, pill boxes, barbed wire or trenches, to say nothing of personnel. [Pg 393]
High Explosive in Projectors. When these are burst by the hundreds on a small area everything movable is blotted out. Thus concrete machine gun emplacements, lookout stations, bomb-proofs and wire entanglements are destroyed, trenches filled up, and the personnel annihilated. This was amply demonstrated on the few occasions when it was actually used at the front. The American Infantry, wherever they saw it tried out, were wild to have more of it used. The German was apparently equally anxious to have the use stopped. It is, however, one of the things that must be reckoned with in the future. It means practically that No Man’s Land in the future will be just as wide as the extreme range of these crude mortars—and here a word of caution. While efforts have been made to increase the range of these mortars, whether of the Livens’ projector or Stokes’ variety, no further increase will be attempted when that increase reduces the speed of firing or the efficiency of the projectile. In other words results depend upon large quantities of material delivered at the same instant on the point attacked, and if this cannot be obtained the method is useless. For this reason these mortars will never be a competitor of the artillery. The artillery will have all that it can do to cover the field within its range—beyond that reached by the mortars.
Phosphorus in 4-Inch Stokes. Phosphorus will be used largely by gas troops, but only in the 4-inch or other Stokes’ mortar that may be finally adopted as best. The Livens’ projector carries too great a quantity, and being essentially a single shot gun, is not adapted to keeping up a smoke screen by slow and continued firing, or of being transported so as to keep up with the Infantry. Phosphorus has also very great value for attacking personnel itself. Any one who has been burned with phosphorus or has witnessed the ease with which it burns when exposed to air, wet or dry, has a most wholesome fear of it. The result of it in the war showed that the enemy machine gunners or other troops would not stand up under a bombardment of phosphorus fired from the 4-inch Stokes’ mortar—each bomb containing about seven pounds.
Thermit. Thermit is used in the same way, and while the idea of [Pg 394] molten metal, falling upon men and burning through clothing and even helmets, is attractive in theory, it proved absolutely worthless for those purposes on the field of battle. It was found impossible to throw sufficiently large quantities of molten metal on a given spot to cause any considerable burn. In other words, the rapid spreading out and cooling of the metal almost entirely ruined its effectiveness, except its effect on the morale. This latter, however, was considerable, as one might judge from seeing the thermit shells burst in air. For this reason thermit may find a limited use in the future.
Height of Gas Cloud. The height to which gas rises in a gas cloud is not exactly known, but it is believed to be not much more than fifty feet, and then only at a considerable distance from the point of discharge. Moving pictures taken of gas clouds show this to be true. It is also indicated by the fact that pigeons, which are very susceptible to poisonous gas, practically always return to their cages safely when liberated in a gas cloud. This was a good deal of a mystery until it was realized that the pigeon escaped through his rising so quickly above the gas. This of course would be expected when it is known that practically all gases successfully used were two or more times as heavy as air. Such gases rise only by slow diffusion, or when carried upwards by rising currents. The absence of these upward currents at night is one of the reasons why gas attacks are more effective at night than during the day.
Horizontal Spread of Gas. Another important thing to know in regard to the behavior of the wave of gas is the horizontal spread of a cloud. If gas be emitted from a cylinder the total spread in both directions from that point is from 20° to 30° or an average of 25°. This varies, of course, with the wind. The higher the wind the less the angle, though the variation due to wind is not as great as might be expected. This horizontal spread of the gas cloud was measured experimentally, and the results checked by aeroplane pictures of heavy wave attacks over the enemy line. In the latter case the path of the [Pg 395] gas was very closely indicated by the dead vegetation. This vegetation was killed and bleached so that it readily showed up in aeroplane photographs. The visibility of a gas cloud arises from the fact that when a large amount of liquid is suddenly evaporated, the air is cooled and moisture condensed, thereby creating a fog. With gases such as mustard gas and others of slight volatility, a visible cloud is not formed. For purposes of identification of points struck by shell, smoke substances are occasionally added, or a few smoke shell fired with the gas shell. As future battle fields will be dotted everywhere with smoke clouds, a point that will be discussed more fully later, the firing of smoke with gas shell will probably be the rule and not the exception.
If we succeed in getting a poisonous gas that has no odor it will be highly desirable to fire it so that it will not be visible. In that case no smoke will be used. Carbon monoxide is such a gas, but there are several important reasons why it has not been used in war. (See page 190). These considerations indicate the general requirements for a successful poisonous gas. If non-persistent it must be quickly volatilized, or must be capable of being driven off by heat or by other means, which can be readily and safely produced in the field. It must be highly poisonous, producing deaths in high concentrations, and more or less serious injuries when taken into the system in quantities as small as one-tenth of that necessary to produce death. If it has a slightly delayed action with no intervening discomfort, it is still better than one that produces immediate discomfort and more or less immediate action. It must be readily compressed into a liquid and remain so at ordinary temperatures, with the pressure not much above 25 or 30 pounds per square inch.
As a persistent gas it must be effective in extremely low concentrations, in addition to having the other qualities mentioned above.
These general characteristics concerning gases apply whether used by Chemical Warfare troops, the Artillery, the Air Service, the Navy, or [Pg 396] the Infantry. In speaking of these substances being used by the Infantry, it is understood that an ample number of Chemical Warfare officers will be present to insure that the gases may not be turned loose when weather conditions are such that the gas might drift back and become a menace to our own troops. This is absolutely essential since no troops who have as varied duties to perform as the Infantry, can be sufficiently trained in the technical side of chemical warfare to know when to put it off on a large scale with safety and efficiency.
The Artillery of the future will probably fire more gas than any other one branch of the Army. There are two reasons for this—first, the large number of guns now accompanying every Army, and second, the long range of many of these guns. As before indicated, the gases are adaptable to various uses, and hence to guns differing both in caliber and range. The gas will be fired by practically all guns—from the 75 mm. to the very largest in use. It is even possible that if guns smaller than the 75 mm. become generally useful that certain gases will be fired by them.
Efficiency of Artillery Gas Shell. It is well to remember in the beginning that all artillery shell so far designed and used, contain only about 10 per cent gas, i.e., 10 per cent of the total weight of shell and gas. It is hoped that gas shell may later be so designed that a somewhat greater proportion of the total weight of the shell will be gas than is now true. This is very desirable from the point of efficiency. As stated above the bombs used by Chemical Warfare troops contain nearly 50 per cent of their total weight in gas, and hence are nearly five times as efficient as artillery shell within the limit of range of these bombs. This fact alone is enough to warrant the use of gas troops to their full maximum capacity in order that the artillery may not fire gas at the ranges covered.
Considering the firing of non-persistent and persistent gases, it may [Pg 397] be said generally that non-persistent gases will be fired only by the medium caliber guns which are available in large numbers. In fact, the firing of non-persistent gases will be confined mainly to the 6-inch or 155 mm. Howitzer and gun.
As our Army was organized in France, and as it is organized at present, the number of 155 mm. guns will be greater than all others put together, except the 75 mm. In order that a non-persistent gas may be most effective a high concentration must be built up very quickly. This necessitates the use of the largest caliber shell that are available in large numbers. Of course, a certain percentage of the gas shell of other calibers may consist of non-persistent gases in order to help out the 155 mm. gun. This is in accordance with the present program for loading gas shell and applies particularly to the 8-inch and 240 mm. Howitzer.
Few Ideally Persistent or Non-Persistent Gases. Naturally there will be very few gases that are ideally non-persistent or ideally persistent. The groups will merge into one another. Those on the border line will be arbitrarily assigned to one group or the other. It might be said definitely, however, that a gas which will linger more than six or possibly eight hours under any conditions, except great cold, will not be considered non-persistent. For reasons of efficiency and economy persistent gases will not be chosen unless they will persist under ordinary conditions for two or three days or more. Accordingly, a gas which would persist for one day only would have to be extraordinarily useful to lead to its adoption.
Firing Non-Persistent Gases. Of the non-persistent gases phosgene is the type and the one most used at present. Furthermore, so far as can now be foreseen, it will continue to be the non-persistent gas most used. It volatilizes very quickly upon the bursting of the shell. Accordingly, in order that the shell fired at the beginning of a gas “shoot,” as they are generally referred to in the field, shall still be effective when the last shell are fired, it is necessary that the whole number be fired within two to three minutes. The temperature and velocity of the wind both affect this. If it be in a dead calm, the time may be considerably extended; if in a considerable wind, it must [Pg 398] be shortened. Another important consideration requiring the rapid firing of non-persistent gases is the fact that nearly all masks thoroughly protect against phosgene and similar gases. It is accordingly necessary to take the enemy unawares and gas him before he can adjust his mask; otherwise, practically no harm will result. From the considerations previously mentioned, these “gas shoots” are usually made at night when, as before stated, carelessness, sleepiness and the resulting confusion of battle conditions always insure more casualties than firing gas in the daytime.
Firing Persistent Gases. The persistent gases will be fired by all caliber guns, but to a less extent by the 155 mm. than by the other calibers. Persistent gases must be sufficiently effective in low concentrations to act more or less alone. If it be desirable to fill an atmosphere over a given area with mustard gas, the firing may extend for two or three, or even five or six hours and all shell still act together. The same is true of bromobenzylcyanide. This, then, permits the minimum number of guns to be used in firing these persistent gases. Inasmuch as they persist and force the wearing of the mask, they are available for use in long-range, large-caliber guns for interdiction firing on cross-roads, in villages, and on woods that afford hiding places, as well as on other similar concentration points.
Firing Irritant Gases. The irritant gases will be fired by the various caliber guns, in the same manner as the persistent and non-persistent gases. We will have non-persistent irritant gases and persistent irritant gases. They are, however, considered as a group because they are used for harassing purposes, due to their efficiency in forcing the wearing of the mask.
Before the signing of the Armistice, the General Staff, A. E. F., had authorized, beginning January 1, 1919, the filling of 25 per cent of all shell with Chemical Warfare materials. The interpretation there given to shell was that it included both shrapnel and high explosive.
Of the field guns in use, the 75 mm. will be best, up to the limit of its range, for persistent gases such as mustard gas, and the tear gas, bromobenzylcyanide. A considerable number, however, were filled with [Pg 399] non-persistent gases and probably will continue to be so, since, due to the very large number of 75 mm. guns available, they can be used to add greatly at times to the amount of non-persistent gas that can be fired upon a given point.
No gas was used by aeroplanes in the World War. Many rumors were spread during the latter part of the war to the effect that the Germans had dropped gas here or there from aeroplanes. Every such report reaching the Chemical Warfare Service Headquarters was run down and in every case was found to be incorrect. However, there was absolutely no reason for not so using gas, except that the German was afraid. In the early days of the use of gas he did not have enough gas, nor had he developed the use of aeroplanes to the point where it would have seemed advisable. When, however, he had the aeroplanes the war had not only begun to go against him, but he had become particularly fearful of gas and of aeroplane bombing.
It does not seem to be generally known, but it is a fact, that after three or four months’ propaganda he made a direct appeal to the Allies to stop the use of gas sometime during the month of March, 1918. This propaganda took the form of an appeal by a Professor of Chemistry who had access to Switzerland, to prevent the annihilation of the Allied forces by a German gas that was to make its appearance in 1918. This German professor claimed that, while favoring the Germans winning the war, he had too much human sympathy to desire to see the slaughter that would be caused by the use of the new gas. The Allies in the field felt that this was simply an expression of fear and that he did not have such a gas. The Germans were accordingly informed that the Allies would not give up the use of gas. Later events proved these conclusions to be absolutely correct. The German evidently felt that the manufacturing possibilities of the Allies would put them in a more predominant position with gas than with anything else. In that he was exactly correct. [Pg 400]
The use of gas by aeroplanes will not differ from its use in artillery or by Chemical Warfare Troops. Non-persistent gases may be dropped on the field of battle, upon concentration points, in rest areas, or other troop encampments to produce immediate casualties. Persistent gases will be dropped particularly around cross-roads, railroad yards, concentration points and encampments that cannot be reached by the artillery. The sprinkling of persistent gases will be one of the best ways for aeroplanes to distribute gas.
It might be said here that the aviation gas bomb will be highly efficient, inasmuch as it has to be only strong enough to withstand the low pressure of the gas and ordinary handling, whereas artillery shell must be strong enough to withstand the shock of discharge in the gun.
When one suggests the possibility of the infantry handling gas, it is at once argued that the infantry is already overloaded. That is true, but in the future, as in the past, the infantryman will increase or decrease his load of a given material just as its efficiency warrants. If he finds that gas will get casualties and help him win victories more readily than an equal weight of any other material, he will carry gas material. A study of the articles of equipment abandoned by 10,000 stragglers in the British Army picked up during the great German drive towards Amiens in March, 1918, illustrates this very clearly. Of the equipment carried by these stragglers, more than 6,000 had discarded their rifles. The helmets were thrown away to a somewhat less extent, but the gas mask had been thrown away by only 800 out of the 10,000. Now the gas mask is not a particularly easy thing to carry, nor was the English type comfortable to wear, but the English soldier had learned that in a gas attack he had no chance whatever of escape if his gas mask failed him. Accordingly, he hung on to the mask when he had discarded nearly everything else in his possession. The same thing will be true of any gas equipment if it proves its worth. [Pg 401]
So far nothing has been said in regard to smoke or incendiary materials. This has been due to the fact that their use is not dependent upon weather conditions to anything near the extent that gas is. Second, the smokes, not being poisonous, are not a danger to our own troops, although they may hamper movements and add to the difficulty of taking a position, if used improperly. Of the two classes of materials, smoke and incendiary, smoke materials may be said to be at least a thousand times as important as the incendiary materials. A material that will burst into flame when a shell is opened or that will scatter balls of burning fire appeals to the popular imagination, and yet actual results achieved by such materials on the field of battle have been almost nil. About the only results worth while achieved by incendiary materials have been in occasionally firing ammunition dumps and more frequently, setting fire to warehouses and other storage places. This will undoubtedly continue in the future.
Of the incendiary materials the least valuable is the flame thrower. In the Chemical Warfare Service it has been the habit for a long while not to mention the flame thrower at all, unless questions were asked about it. It is mentioned here to forestall the questions. Even the German, who invented it and who, during the two years of trench warfare, had full opportunity for developing its use, finally came to using it largely as a means of executing people that he did not want to shoot himself. Men falling in that class were equipped with flame throwers and sent over the top. The German knew, as did the Allies, that each man with a flame thrower became a target for every rifle and machine gun nearby. The flame thrower is very quickly exhausted and then the one equipped with it has no means of offensive action, and in addition, is saddled with a heavy load, hampering all movements, whether to escape or to advance. [Pg 402]
There will probably be some use for materials such as metallic sodium, spontaneously inflammable oils, etc., that will burst into flame and burn when exposed to the air, though white phosphorus is probably equal, and in most cases vastly superior to anything else so far suggested. Phosphorus burns with an unquenchable flame when exposed to the air, whether wet or dry. It is of great value for screening purposes, and for use against the enemy’s troops. The German did not use phosphorus simply because he did not have it, just as he did not use helium in his observation balloons because he did not have it.
The value of phosphorus was just beginning to be realized slightly when the Americans entered the war, while its full value was not appreciated even by the American troops when the war closed.
The work of the First Gas Regiment with phosphorus against machine gun nests proved how valuable it is against the enemy’s troops. It proved also its tremendous value as a screen.
The Chemical Warfare Service was prepared to fill a great number of artillery shell with phosphorus, but due to the failure of our shell program to mature before the Armistice, phosphorus was not used by American artillery to any appreciable extent.
Smoke will be used by every fighting arm of the Service in practically every battle, both by day and by night. If you have ever tried on a target range to shoot at a target that was just beginning to be obscured by a fog, you will recognize the difficulty of hitting anything by firing through an impenetrable smoke screen. It is simply a shot in the dark. Future battles will witness smoke formed by smoke candles that are kept in the trenches or carried by the troops, by smoke from bursting artillery shell and rifle grenades, by smoke from aeroplane bombs and possibly even from what is known as the smoke [Pg 403] knapsack. The knapsack produces a very dense white smoke and very economically, but will probably not be much used. This is because, notwithstanding its efficiency, the knapsack cannot be projected to a distance, that is, the smoke screen is generated on the person carrying the knapsack. On the other hand the great value of phosphorus is that it can be fired to great distances in rifle grenades or artillery shell, and dropped from aviation bombs. The smoke screen is thus established in front of the object it is desired to cut off, whether it be a battery of artillery, an advancing wave of infantry, or a lookout station. Thus smoke, for screening purposes alone, will be used to a tremendous extent. It will also be used in conjunction with gas.
Due to the smoky appearance of an ordinary gas cloud and to the coming use of poisonous smokes, no one on the field of battle in the future will ever be certain that any given smoke cloud is not also a poisonous cloud until he has actually tested it. And there lies an opportunity for the most intense study and for the greatest use of the proverbial American ingenuity that war has ever furnished.
In the variations that can be played with smoke containing gas, or not containing gas, with smoke hurled long distances by the artillery or dropped from aeroplanes, the possibilities indeed are unlimited. Every officer will need to study the possibilities of smoke, both in its use against him and in his use of it against the enemy. He can probably save more casualties among his own troops by the skillful use of smoke than by any other one thing at his command. On the other hand, the unskilled use of smoke on the part of one side in a battle may lead to very great casualties in proportion to those of the enemy should the latter use his smoke skillfully. This is a subject that deserves deep and constant study.
Smoke in the future will be the greatest protective device available to the soldier. It shuts out not only the view in daylight, but the [Pg 404] searching of ground at night by searchlights, by star bombs or other means for illuminating the battlefield. It has already been used extensively by the Navy and undoubtedly will be used far more extensively in the future.
Modern artillery shell have distinctive colors for high explosive, for shrapnel, for incendiary materials, and for gases. A grayish color has been adopted as the general color of the paint on all gas shells, bombs and cylinders. In addition a system of colored bands has been adopted. These bands are white to indicate poisonous non-persistent substances, and red—persistent. Yellow is used to indicate smoke. With any given combination of red and white and yellow bands, the artillery-man at the front can tell, at a glance, whether the gas is non-persistent or whether it is persistent, and also whether or not it contains smoke. There will be secondary markings on each shell which, to the trained Chemical Warfare Service officer, will indicate the particular gas or gases in the shell. These markings however, will be inconspicuous and no attempt will be made to give the information to the soldier or even to the average officer firing gas.
These secondary markings are for the purpose of enabling the Chemical Warfare Service officers in charge to use certain gases for particular uses in those comparatively rare cases when sufficient gas is on hand and sufficient time available to enable such a choice to be made.
[Pg 405]
(From the Field Point of View)
The best defense against any implement of war is a vigorous offense with the same implement. This is a military axiom that cannot be too often, or too greatly emphasized, though like other axioms it cannot be applied too literally. It needs a proper interpretation—the interpretation varying with time and circumstances. Thus in gas warfare, a vigorous offense with gas is the best defense against gas. This does not mean that the enemy’s gas can be ignored. Indeed, it is more important to make use of all defensive measures against gas than it is against any other form of attack. Gas being heavier than air, rolls along the ground, filling dugouts, trenches, woods and valleys—just the places that are safest from bullets and high explosives. There it remains for hours after it has blown away in the open, and, since the very air itself is poisoned, it is necessary not only that protection be general but that it be continuous during the whole time the gas is present.
The earliest protection against gas was the crudest sort of a mask. The first gas used was chlorine and since thousands of people in civil life were used to handling it, many knew that certain solutions, as hyposulfite of soda, would readily destroy it. They also knew that if the breath could be drawn through material saturated with those solutions, the chlorine would be destroyed. Thus it was that the first masks were simple cotton, or cotton waste pads, which were dipped into hyposulfite of soda solutions and applied to the mouth and nose during [Pg 406] a gas attack. These pads were awkward, unsanitary, and, due to the long intervals between gas attacks, were frequently lost, while the solution itself was often spilled or evaporated. The net result of all this was poor protection and disgust with the so-called masks.
After using these, or similar poor excuses for a mask, for a few weeks, the British designed what was known as the PH helmet. In a gas attack the sack was pulled over the head and tucked under the blouse around the neck, the gas-tight fit being obtained by buttoning the blouse over the ends of the sack. This PH helmet was quite successful against chlorine and, to a much less extent, against phosgene, a new gas introduced during the spring of 1916.
But it was warm and stuffy in summer—the very time when gas is used to the greatest extent—while the chemicals in the cloth irritated the face and eyes, especially when combined with some of the poisonous gases.
Probably as a result of experience with oxygen apparatus in mine rescue work, Colonel Harrison suggested making a mask of which the principal part was a box filled with chemicals and carried on the chest. A flexible tube connected the box with a mouthpiece of rubber. Breathing was thus through the mouth and in order to insure that no air would be breathed in through the nose, a noseclip was added.
This, of course, cared for the lungs, but did not protect the eyes. Their protection was secured by making a facepiece of rubberized cloth with elastics to hold it tight against the face. The efficiency of this mask depends, then, first upon the ability of the facepiece to keep out lachrymatory gases which affect the eyes, and, second, upon a proper combination of chemicals in the box, to purify the air drawn into the lungs through the mouthpiece. (Details are given in Chapter XII).
While the charcoal and soda-lime granules furnished an adequate protection against all known true gases, they did not furnish [Pg 407] protection against certain smokes or against minute particles of liquid gas. Since certain smokes, as stannic chloride, though not deadly, are so highly irritating as to make life unbearable, it early became necessary to devise means for keeping them from going through the masks. This was done in the first masks by adding a sufficient thickness of cotton batting. The cotton was usually placed in three layers alternating with the charcoal and granules, as it was thought the latter would be held in place better by that means.
Some time after stannic chloride came into use the Germans started firing shells containing a small quantity of diphenylchloroarsine, popularly known as “Sneezing Gas.” Protection against this is discussed in Chapter XVIII.
When it became necessary, with the creation of a Chemical Warfare Service in France in August, 1917, to decide upon a mask for American troops, there were available for purchase two types—the British type and the French M-2. The French M-2 consisted essentially of 32 layers of cloth impregnated with various chemicals, through which the air was breathed both in and out. This mask was quite effective against ordinary field concentrations of most gases, but was utterly inadequate to care for the high concentration of phosgene obtained in the front line from cloud gas or from projector gas attacks. It was also poor against chloropicrin. The M-2 was, however, very light and easy to carry and moreover was deemed sufficient to protect against concentrations of cloud gas even, at points more than five miles distant from the front line.
Furthermore, it was felt desirable at first to have an auxiliary or emergency mask in addition to the principal one, for use in case the principal mask was worn out or damaged. Accordingly both types of masks were adopted and the day after Fries took charge of the Chemical Warfare Service, A.E.F., on August 22, 1917, 100,000 of each were purchased, although there were then only ten or twelve thousand American troops in France requiring masks. Later additional masks of both kinds were purchased to tide over the American troops until a sufficient quantity of the British type masks could be manufactured in [Pg 408] the United States. The total of British masks purchased amounted to about 700,000.
However, within a comparatively short time after American troops got into the front line it was realized that a second mask, inferior in protection to the first, was highly undesirable. During a gas attack men seemed to acquire an uncontrollable desire to shift from one mask to the other. This shifting in nearly every case resulted in a casualty. We then came rapidly to the conclusion that one mask only should be furnished, and that one the best that could be made, and then to impress upon the soldier the fact that his life depended upon the care he took of his mask. This proved to be an entirely sound conclusion, as the number of men gassed through injuries to the mask was comparatively small. An interesting proof of the value the soldier placed upon his mask was shown by the articles of equipment thrown away by 10,000 British stragglers in the great German offensive of March, 1918. Of the articles thus thrown away the gas mask came at the foot of the list, with only 800 missing. The steel helmet is said to have come next with about 4,000 missing.
When adopting the British respirator in August, 1917, it was decided that the American face as well as the American stature was probably larger than the English. Accordingly inquiry was made in regard to the sizes of masks issued to the Canadians as it was thought probable they required a greater proportion of the larger size masks than did the English. When prescribing the relative quantities of each size of mask to be furnished Americans, the Canadian requirements were taken as a base but with the larger sizes increased slightly over the Canadian requirements. As a matter of fact even these increases proved considerably too small, so that the numbers in the two sizes above normal had to be finally more than doubled.
The American Gas Service felt from the beginning that a design which [Pg 409] attached the box of chemicals to the facepiece was unsound in principle (this design was used in the German mask and in the French A. R. S. masks), since it did not allow proper flexibility for increasing the size of the box to care for new gases. Furthermore, the weight of the box during movement caused the facepiece to swing slightly from side to side. This interfered with vision and tended to lift the facepiece away from the face and allow gas to enter. That the objections of the American Gas Service to this type were correct was proved by the difficulty encountered toward the end of the war by both the French and the Germans in trying to provide a suitable filter for protection against particulate clouds and the smokes, such as stannic chloride and diphenylchloroarsine.
As between the mask and poisonous gases, we have the old struggle of the battleship armor against the armor-piercing projectile. While the armor-piercing projectile has always had a little the better of the game, it is just the reverse with gases. The gas mask has always been just a little better than the gases, so that very few casualties have occurred through failure of the mask itself. This margin of safety has never been any too great, and that we have had a margin at all is due to the energy, skill and enthusiasm of those developing and manufacturing masks in England, France, and particularly in the United States.
However, the mask at the best is uncomfortable, causes some loss of vigor, and even with the very best American masks there is some loss in vision. The wearing effect on troops results mostly from the increased resistance to breathing. Accordingly a tremendous amount of study and effort was made to decrease this breathing resistance. In the English type masks this resistance was equal to the vacuum required to raise a column of water about four and one-half inches. Adding the sulfite paper to protect against diphenylchloroarsine increased this resistance by about one inch. This put a heavy burden on the wearer of the mask whenever it was necessary for him to do any manual labor while wearing it. In addition earlier masks left a good deal to be desired in the way of reducing resistance by proper sized tubes, angles and valves through [Pg 410] which the air was drawn. This was much more easily overcome than reducing the resistance through the chemicals and charcoal and the materials for protection against diphenylchloroarsine. In the latest type canister, devised after long trials for the American forces, this resistance was brought down to about two inches of water. What this reduction in resistance means no one knows except one who has worn the old mask with its mouthpiece and four to six inches’ resistance and has then replaced that mask for one through which he breathes naturally with only two inches’ resistance.
The American Gas Service felt from the beginning that the mouthpiece and noseclip must be abandoned and bent every effort toward getting a mask perfected for that purpose. The English opposed this view fiercely for nearly a year. This position on the part of the English was more or less natural. They developed their mask in the beginning for protection against cloud gas. In those days the opposing trenches were close together. Moreover, front line trenches were quite strongly manned. The result was that a large number of men were exposed to a very high concentration of gas, but—and highly important—for a short period only. Inasmuch as the German feared this cloud gas even more than the English there was no danger of his attacking in it. The English rules of conduct during a gas attack called for all movement to stop and for every man to stand ready until the cloud passed. Accordingly, the man was breathing the easiest possible and hence did not suffer particularly from the resistance.
With the advent of mustard gas, however, the whole general scheme of protection changed. Mustard gas, as is well known, is effective in extremely low concentrations and has very great persistency. In dry warm weather mustard gas, scattered on the ground and shrubbery, will not be fully evaporated for two to three days and accordingly will give off vapors that not only burn the lungs and eyes but the soft, moist parts of the skin as well. In cool, damp weather the gas remains in dangerous quantity for a week and occasionally longer. Since this gas, in liquid form, evaporates too slowly for use in gas clouds, it is used [Pg 411] altogether in bombs and shells. Accordingly it could be expected to be and actually is fired at all ranges from the front line to nearly eight miles back of that line. Hence, with the coming of mustard gas, the need for protection changed from high protection for a short period to moderate protection for very long periods. Indeed, mustard gas makes it necessary for men to wear masks just as long as they remain in an area infected with it. There is still occasional need for high protection for short periods, but with the increase in the efficiency of charcoal alone, it is found that the amount of charcoal and chemicals in the canister can be very greatly reduced and still maintain sufficient protection for the high concentrations encountered in cloud gas and projector attacks.
It seems physically impossible for the ordinary man to wear the British mask with its mouthpiece and noseclip more than six to eight hours and vast numbers are unable to even do that. How many thousands of casualties were suffered through men losing their mental balance from exhaustion and the discomfort of the mouthpiece and noseclip no one knows. Such men tore off the mask, stating that they would rather die than endure the torture of wearing it longer. Furthermore, the poor vision of this mask led to the habit of taking the facepiece off while still leaving the mouthpiece and noseclip in place. This gave protection to the lungs, but exposed the eyes, and as mustard gas affects the eyes very readily this alone led to thousands of casualties. There was another interesting side to this situation. The malingerer who wanted to get out of the front line and was willing to take any action, however cowardly, to achieve that end, deliberately removed the facepiece and thus suffered gassing of the eyes. The effect of mustard gas soon became so well known that the malingerer knew gassing of the eyes never resulted in death or permanent loss of sight. With the new type of American mask, the protection of eyes and lungs depends solely upon the fit around the face and no such playing with the mask can be done. [Pg 412]
Without going into further details in regard to masks it is sufficient to state that at the end the Americans had produced a mask thoroughly comfortable, giving complete protection against gases and smoke clouds, and one that was easy to manufacture on the huge scale (fifty to seventy-five thousand per day) which was necessary to provide masks for an army of three to four million men in the field.
Napoleon is credited with saying “In order to make an omelet, it is necessary to break some eggs.” Every student of war realizes that casualties cannot be avoided in battle and yet one American Staff Officer went so far as to refuse to use gas offensively unless the Chemical Warfare Service could absolutely guarantee that not a single American casualty could occur under any circumstances. This same idea early got into the heads of the laboratory workers on masks. They seemed to feel that if a single gas casualty occurred through failure of the mask, their work would be a failure or at least they would be open to severe criticism. Accordingly efforts were made to perfect masks and to perfect protection regardless of the discomfort imposed upon the wearer of the mask. This idea was very difficult to eradicate. The laboratory worker who accustoms himself to experiment with a particular thing forgets that he develops an ability to endure discomfort, that is not possible of attainment by the ordinary man in the time available for his training.
Furthermore, if the need for such training can be avoided it is of course highly desirable. This applies to the mouthpiece of the British respirator; to elastics that cause undue discomfort to the face; to the noseclip and to the large boxes that cause too great resistance to breathing.
It may be taken as a general rule that when protection requires so much effort or becomes so much of a burden that the average man cannot or will not endure it, it is high time to find out what the average man will stand and then provide it even if some casualties result. Protection in battle is always relative. A man who cannot balance protection against legitimate risk has no business passing on arms, equipment or tactics to be used in battle. [Pg 413]
Bitter experience taught the Allies as well as the Americans that no matter how efficient the gas mask and other defensive appliances, they would not take the place of thorough and constant training. One of the greatest difficulties at first was to get American troops to realize that a thing as invisible as gas, with in many cases no offensive smell and producing no immediate discomfort, could be deadly. Nothing but constant drill and constant reiteration of these dangers could get this fact impressed on them. Indeed it never was impressed sufficiently in any of the earlier divisions of American troops in the line to prevent their taking such chances that each division suffered heavy loss on one or more occasions from gas attacks.
A great deal of emphasis had been placed by the English upon the adjustment of the mask in the shortest possible time, this time having been officially set at six seconds after the alarm. The Americans in adopting the mask in toto naturally had to adopt the rules for adjusting it and wearing it. Experience, however, taught them in a few months that the effort to attain too great speed was dangerous. It tended to rattle the soldier and to result in poor adjustment of the mask, both of which led to casualties. Accordingly in the latest instructions for defense against gas all reference to six seconds was eliminated and emphasis placed on the necessity of accurate adjustment of the mask. Inasmuch as any man, practically without effort or previous drill, can hold his breath for twenty seconds, the need for great speed in adjusting the mask is not apparent.
The first regulations and those in general use up to near the close of hostilities, prescribed that the soldier should hold his breath and adjust his mask. It seemed impossible to overcome the natural inference that “holding the breath” meant first the drawing of a full breath. This was obviously highly dangerous if gas were actually present before the alarm was heard, as was often the case with projector and artillery [Pg 414] gas shell attacks. The change was then made to the phrase “Stop Breathing and Stay Stopped until the Mask is Carefully and Accurately Adjusted.”
While the importance of impressing upon the soldier the danger of gas was early appreciated it was deemed necessary not to make him unduly afraid of the gas. However, as gas defense training in our Army got a big start over gas offense training, this became a matter of very great importance. In fact, due to a variety of causes, training in the offensive use of gas was not available for any troops until after their arrival in France. This resulted in officers and men looking upon the gas game, so far as they were individually concerned, as one of defense only. Accordingly after their arrival in France it became very difficult not only to get some of our officers to take up the offensive use of gas but even to get them to permit its use along the front they commanded.
Notwithstanding all the care taken in training Americans in gas defense there arose an undue fear of the gas that had to be overcome in order to get our troops to attack close enough to their own gas to make it effective. This applied to the use of gas by artillery as well as to its use by gas troops. However, it should be said that in every instance where gas was once used on an American front all officers in the Division, or other unit, affected by it were always thereafter strongly in favor of it.
The Germans also had serious troubles of their own over the psychology of gas training. As stated elsewhere they were using mustard gas nearly eleven months before the Allies began using it. During that time, for purposes of morale, if not sheer boastfulness, the Germans told their men that mustard gas could not be made by the Allies; that it was by far the worst thing the war had produced—and in that statement they were correct—and that they would win the war with it—in which statement they were far from correct. When the Allies began sending it [Pg 415] back to them they had to reverse their teachings and tell their men that mustard gas was no worse than anything else, that they need not be afraid of it and that their masks and other protective appliances gave full protection against it. They thus had a problem in psychology which they never succeeded in fully solving. Indeed there is no question but that the growing fear of gas in the minds of the German is one of the reasons that prompted him to his early capitulation.
In the early days it was very difficult to get officers to realize the absolute necessity of night drill in the adjustment of the mask. For various reasons, including surprise, gas attacks were probably eighty to ninety per cent of the time carried out at night. Under such conditions confusion in the adjustment of the mask is inevitable without a great deal of practice before hand, especially for duty in trenches with narrow spaces and sharp projecting corners. There are numerous instances of men waking up and getting excited, who not only gassed themselves, but in their mad efforts to find their masks, or to escape from the gas, knocked others down, disarranging their masks and causing the gassing of from one to three or four additional men. The confusion inherent in any gas attack was heightened in the latter stages of the war by heavy shrapnel and high explosive bombardments that accompanied nearly all projector and cloud gas attacks for that very purpose. The bombardment was continued for three or four hours to cause exhaustion and removal of the mask and to prevent the removal of the gassed patients from the gassed area.
Efforts were made by the enemy and by all the Allies throughout the war to invent a mechanical detector that would show when gas was present in dangerous quantities. While scores, perhaps hundreds, of these were invented none proved simple, quick, or certain enough in action to make their adoption desirable. In every case it was necessary to rely on the [Pg 416] sense of smell. Thus it was that as the war wore on, more and more attention was given to training officers and non-commissioned officers to detect various kinds of gases in dangerous quantities by the sense of smell.
In the American Gas Defense School for officers this was done wholly by using captured German gases. This was because certain gases have quite different smells, depending upon the impurities in the gas and also upon the solvents sometimes mixed with them. Thus the German mustard gas has a mustard smell, while the Allies mustard gas, due to a slight difference in the method of manufacture, has a very perfect garlic odor. Not only must officers and men who handle gas training know the smell of the various gases, but they must know when the concentration of each is high enough to be dangerous. This is not easy to learn because the strength of the various gases in dangerous concentrations varies through wide limits. Not only does the strength of the gases vary and the sharpness of the odors accordingly, but the mingling of poisonous gases with other gases from high explosive and shrapnel tends to obscure these odors and make them more difficult of detection.
A great deal of thought was given toward the end of the war to the subject of deceptive gases which could by powerful or peculiar odors mask the dangerous gases. This masking was to deceive the enemy when dangerous gases were present or to admit an attack without masks while the enemy was wearing his through thinking there was a dangerous gas when as a matter of fact none existed.
In gas warfare, the German, as well as the Allies, was exercising his ingenuity in devising new and startling methods of making gas attacks. A well known trick with the German was to fire gases for several days, particularly against green troops, in concentrations so slight as to do no harm. When he felt that he had lulled those troops to a sense of the ineffectiveness of his gas, he sent over a deadly concentration. In spite of the warning that this was what was happening, he often [Pg 417] achieved too great a success. Before the war closed, however, the American was beginning to out-think and out-wit the German in this method of warfare.
With the advent of mustard gas which burned the body, a new and serious difficulty in protection arose. At first it was thought mustard gas burned only when the liquid from the bursting shell actually splashed on the clothing or skin. This was unfortunately soon found to be not true. The gas itself rapidly penetrates clothing and burns the skin even when the concentration of the gas is very low. Probably the majority of burns from mustard gas arose from concentrations of gas consisting of less than one part of gas to five hundred thousand of air. Furthermore, the gas is fully fifty per cent cumulative in its effects, that is, in extremely low concentrations over a period of hours it will produce more than fifty per cent the effect that a far higher concentration would produce in a relatively shorter time.
The Allies were not long in discovering that oilcloth afforded very complete protection against mustard gas. The ordinary oilcloth, however, was too thick, too hot and too heavy for general use. Experiments soon showed that cloth thoroughly impregnated with boiled linseed oil would give protection. In order to make this protection more perfect a certain amount of paraffin was added. All this made the clothing air-tight, rather stiff and always uncomfortable. Notwithstanding these discomforts, hundreds of thousands of oiled suits, and as many pairs of oiled gloves were made and issued to artillery troops, and to troops especially charged with handling mustard gas shells, or to those employed in destroying mustard gas in shell holes by spreading chloride of lime over them.
The importance of protection against mustard gas burns led to extensive researches being made with a view to finding a cloth which would be comfortable and porous and while stopping mustard gas would yet be sufficiently durable and comfortable to be issued to infantry troops as well as to artillery and other special troops. This, it is understood, [Pg 418] had been achieved, just prior to the Armistice. Still more desirable would be the discovery of a chemical substance which could be applied to all uniforms and Army clothing and thus protect the regulation clothing against the penetration of mustard gas, and thereby avoid carrying extra clothing for that special purpose.
As soon as it was fully realized that mustard gas persisted for several days it was decided to run complete reliefs of men into and out of areas that had been heavily shelled with mustard gas, or better still, where practicable, to completely evacuate the area. Inasmuch as the gas is dangerous to friend and foe alike, this method was comparatively safe and was used to a very considerable extent. With the warfare of movement that existed over most of the active front throughout the season of 1918, this moving of troops out of infected areas became highly important and, when skillfully done, often resulted in a great saving of troops and at the same time prevented the enemy from receiving any particular tactical advantage from his mustard gas attacks.
There was one very excellent example of this a few miles to the northwest of Château-Thierry prior to the counter-offensive of July 18, 1918. At that time the Germans heavily shelled with mustard gas four or five small woods and two or three villages. It was necessary for the men to stay in these woods during the day, as they afforded the only protection obtainable from machine guns, shrapnel and high explosive. At the time this occurred American gas officers generally understood the necessity of getting troops out of a mustard gas infected area. Accordingly all began searching for places safe from the mustard gas. In one particular instance the gas officer of a regiment discovered that a portion of the woods his men were in was free from the gas, and the regimental commander, promptly following his advice, moved his troops into the free area. As a result of this prompt action the regiment had only four light gas casualties, although all told there [Pg 419] were several hundred mustard gas casualties in this attack, the number per thousand generally being from ten to twenty times that of the thousand men just mentioned.
On this as well as other occasions the Germans fired some diphosgene and Blue Cross (Sneezing gas), as well as mustard gas. This added to the difficulty of determining areas free from the latter. In the future such mixing of poisonous gases may always be expected and, in addition, gases which have no value other than that of masking the poisonous ones will be fired. While with practically all gases except mustard gas a man is comparatively safe while breathing a concentration very noticeable to the sense of smell, the only safe rule with mustard gas is to consider as dangerous any concentration that can be smelled.
For the reason that this gas persists longer in calm areas, woods are always to be avoided, where practicable, and also, since all gases, being heavier than air, tend to roll into depressions and valleys, they should be avoided. There have been a number of authentic cases where batteries in hollows or valleys suffered severely from mustard gas, while troops on nearby knolls or ridges were comparatively free, though the difference in the amount of shelling of the two places was not noticeable.
Of great importance with all gases is the posting of a sufficient number of sentries around men sleeping within the range of gas shell. The worst projector gas attack against the Americans was one where the projectors were landed among a group of dugouts containing men asleep without sentries. The result was a very heavy casualty list, coupled with a high death rate, the men being gassed in their sleep before they were awakened.
Prior to the introduction of mustard gas all that was necessary to get rid of gas was to thoroughly ventilate the spot. Thus in trenches and dugouts, fires were found to be very efficient, simply because they [Pg 420] produced a circulation of air. In the early days, among the British, the Ayrton fan, a sort of canvas scoop, was used to throw the gas out of the trenches. While this was taken up in the American Service, it did not become very important, since it was found that, under ordinary atmospheric conditions, natural ventilation soon carried the gas out of the trench proper, while fires in dugouts were far more efficient than the fans. Likewise the Ayrton fan smacked too much of trench warfare which had reached a condition of “stalemate”—a condition that never appealed to the Americans and a condition that it is hoped never will.
With mustard gas, however, conditions were entirely changed. This liquid having a very high boiling point and evaporating very slowly, remains for days in the earth and on vegetation and other material sprinkled with it. This was particularly true in shell holes where the force of the explosion drove the gas into the earth around the broken edges of the hole. While many substances were experimented with, that which proved best and most practical under all conditions, was chloride of lime. This was used to sprinkle in shell holes, on floors of dugouts and any other places where the liquid might be splashed from bursting shells. It was also found very desirable to have a small box of this at the entrance to each dugout, so that a person who had been exposed to mustard gas could thoroughly coat his shoes with it and thus kill the mustard gas that collected in the mud on the bottom and sides of his shoes.
There are many instances where the occupants of dugouts were gassed from the gas on the shoes and clothing of men entering the dugout. Not only were occupants of dugouts thus gassed but a number of nurses and doctors were gassed while working in closed rooms over patients suffering from mustard gas poisoning. Even under the conditions of warfare existing where the Americans were generally in action, the quantity of chloride of lime required amounted to several hundred tons [Pg 421] per month which had to be shipped from the United States. Chloride of lime was also very convenient to have at hand around shell dumps for the purpose of covering up leaky shells, though rules for handling mustard gas shells usually prescribed that they be fired and where that was not practicable to bury them at least five feet under the surface of the ground. This depth was not so much for the purpose of getting rid of the gas as it was to get the shell so deep into the ground that it would not be a danger in any cultivation that might later take place.
Much was learned toward the end of the war about ways of getting through or around areas infected with mustard gas. For instance, if mustard gas be fired when the weather is in the neighborhood of freezing or somewhat below, it will remain on the ground at night with so little evaporation as not to be dangerous. The same will be true during the day time if the weather is cloudy as well as cold. If, however, the days are bright and the nights cold, mustard gassed areas can be safely crossed by troops at night provided care is taken in brush and bushes to protect the feet and clothing from the liquid splashed on bushes. If the sun comes out warm in the morning such areas may be quite dangerous for three to four hours following sun-up and indeed for the greater part of the day. Quite a large number of casualties were ascribed to this fact in the heavy attack on the British front west of Cambrai just prior to the great German drive against Amiens, March 21, 1918.
Since mustard gas has a greatly delayed action it was found that if men who had been exposed to it could be given a thorough bath with soap and water within a half hour or even a full hour, the mustard gas burns would be prevented or very greatly reduced in severity. Accordingly degassing units were developed consisting essentially of a 5 ton truck with a 1200 gallon water tank, fitted with an instantaneous heater and [Pg 422] piping to connect it to portable shower baths. Another truck was kept loaded with extra suits of underclothing and uniforms. These degassing units were to be provided at the rate of two per division. Then, in the event of a mustard gas attack anywhere in the division, one of these units would be rushed to that vicinity and the men brought out of the line and given a bath and change of clothing as soon as possible. At the same time they were given a drink of bicarbonate of soda water and their eyes, ears, mouth and nasal passages washed with the same.
It was very early learned that mustard gas, or minute particles of the liquid gas settling on food, caused the stomach to be burned if the food were eaten, just as the eyes, lungs and skin of the body are burned from gas in the air. This made it necessary then to see that all food liable to exposure to mustard gas attacks was protected, and tarred paper for box linings or tops was found by the Gas Service to furnish one of the cheapest and most available means of doing this.
Numerous, indeed, were the devices invented at one time or another with which to sound gas alarms. The English early devised the Strombos horn, a sort of trumpet operated by compressed air contained in cylinders carried for that purpose. Its note is penetrating and can be heard, under good conditions, for three or four miles. When cloud gas attacks, which occurred only at intervals of two to four months, were the only gas attacks to be feared, it was easy enough to provide for alarm signals by methods as cumbersome and as technically delicate as the Strombos horn.
With the advent of shell gas in general, and mustard gas in particular, the number of gas attacks increased enormously. This made it not only impossible, but inadvisable also, to furnish sufficient Strombos horns for all gas alarms, as gas shell attacks are comparatively local. In such cases, if the Strombos horn is used to give warning, it causes troops who are long distances out of the area attacked to take [Pg 423] precautions against gas with consequent interference with their work or fighting.
To meet these local conditions metal shell cases were first hung up and the alarm sounded on them. Later steel triangles were used in the same way. At a still later date the large policeman’s rattle, well known in Europe, was adopted and following that the Klaxon horn. As the warfare of movement developed the portability of alarm apparatus became of prime importance. For those reasons the Klaxon horn and the police rattle were having a race for popularity when the Armistice was signed.
A recent gas alarm invention that gives promise is a small siren-like whistle fired into the air like a bomb. It is fitted with a parachute which keeps it from falling too rapidly when the bomb explodes and sets it free. Its tone is said to be very penetrating and to be quite effective over an ample area. Since future gas alarm signals must be efficient and must be portable, the lighter and more compact they can be made the better; hence the desirability of parachute whistles or similar small handy alarms.
One of the problems that remained unsolved at the end of the war was how to determine when to issue new boxes, or canisters, for masks. One of the first questions asked by the soldier is how long his mask is good in gas, and how long when worn in drill where there is no gas. This information is of course decidedly important. Obviously, however, it is impossible to tell how long a canister will last in a gas attack, unless the concentration of gas is known—that is, the life of the box is longer or shorter as the concentration of gas is weak or heavy.
A realization of this need led mask designers to work very hard, long before the necessity for comfort in a mask was as fully realized as it was at the end of the war, to increase the length of life of the canister. To get longer life they increased the chemicals and this in turn increased the breathing resistance, thereby adding to the [Pg 424] discomfort of the soldier when wearing the mask. Finally, however, it was found that in the concentration of gas encountered on an average in the field, the life of the comparatively small American boxes was sufficient to last from fifty to one hundred hours, which is longer than any gas attack or at least gives time to get out of the gassed area.
The British early appreciated the necessity of knowing when boxes should be replaced. They accordingly devised the scheme of furnishing with each mask a very small booklet tied to the carrying case in which the soldier could not only enter a complete statement of the time he had worn the mask but also the statement as to whether it was in gas or for drill purposes only. The soldier was then taught that if he had worn the mask, say for forty hours, he should get a new box. But the scheme didn’t work. In fact, it was one of those things which foresight might have shown wouldn’t work. Indeed, any man who in the hell of battle can keep such a record completely, should be at once awarded a Distinguished Service Medal.
As gas warfare developed not only were all kinds of gas shells sent over in a bunch but they were accompanied by high explosive, shrapnel and anything else in the way of trouble that the enemy possessed. A man near the front line, under those conditions, had all he could do and frequently more than he could do, to get his mask on and keep it on while doing his bit. Consequently he had no time, even if he had the inclination, to record how long he had the mask in the various gases.
In this connection, after the Armistice was signed we in the field were requested to obtain for experimental purposes 10,000 canisters that had been used in battle. Each was to be labeled with the length of time it had been worn in or out of gas, and if in gas, the name of each gas and the time the mask was worn in it. This request is just a sample of what is asked by those who do not realize field conditions. One trip to the front would have convinced the one making the request of the utter impossibility of complying with it, for really no man knows how long he wears a mask in gas. With gas as common and as difficult to detect [Pg 425] (when intermingled with high explosive gases and other smells of the battle field) as it was at the end of the war, each man wore the mask just as long as he could, simply as a matter of precaution.
Before hostilities ceased we were trying out a method of calling in say fifty canisters per division once a week for test in the laboratory. If the tests showed the life of the canisters to be short new canisters would be issued. While we did not have opportunity to try out this plan, it gave promise of being the best that could be done. With gas becoming an every day affair, the only other alternative would seem to be to make issues of new boxes at stated intervals. On the other hand there are no definite records of casualties occurring from the exhaustion of the chemicals in the box. Undoubtedly some did occur, but they were very, very few. In nearly all cases the masks got injured, or the box became rusted through before the chemicals gave out.
It will probably be a shock to most people to learn that with more than two million men in France we required nearly 1500 tons of gas material per month. This tonnage was increasing, rather than decreasing, to cover protective suits, gloves, pastes, and chloride of lime, as well as masks. The British type respirator was estimated to last from four to six months. The active part of the war, in which the Americans took part, was too short to determine whether this was correct or not. The indications were, however, that it was about right, considering rest periods and fighting periods.
With the new American mask, with its much stronger and stiffer face material, the chances are that the life will be considerably increased although the more constant use of the mask will probably offset its greater durability. A longer life of mask would of course be a decided advantage as it would not only reduce tonnage, but would reduce manufacturing and distribution as well. The estimates on which we were working at the end looked forward to requiring from the United States [Pg 426] about one-third pound per man per day for all troops in France, in order to keep them supplied with gas defense material and with the gases used offensively by gas troops. All gas shell, hand grenades, etc., used by other than gas troops required tonnage in addition to the above.
In summing up then, it is noted that there are several important things in defense against gas. First, the mask which protects the eyes and the lungs. Second, the training that teaches the man how to utilize to best advantage the means of protection at his disposal, whether he be alone or among others. Third, protective clothing that protects hands and feet and the skin in general. Fourth, a knowledge of gases and their tactical use that will enable commanders, whenever possible, to move men out of gas infected areas. Fifth, training in the offensive use of gas, as well as in defensive methods, to teach the man that gas has no uncanny power and that it is simply one element of war that must be reckoned with, thus preventing stampedes when there is really no danger.
While these are the salient points in defense against gas, above them and beyond them lies the vigorous offensive use of gas. This involves not only the research, development and manufacture of necessary gases in peace time, but also the necessary training to enable our nation to hurl upon the enemy on the field of battle chemical warfare materials in quantities he cannot hope to attain.
[Pg 427]
“Peace hath her victories no less renowned than war.” Thus runs the old proverb. In ancient times war profited by peace far more than peace profited by war if indeed the latter ever actually occurred. The implements developed for the chase in peace became the weapons of war. This was true of David’s sling-shot, of the spear and of the bow. Even powder itself was probably intended and used for scores of years for celebrations and other peaceful events.
The World War reversed this story, especially in its later phases. The greater part of the war was fought with implements and machines prepared in peace either for war or for peaceful purposes. Such implements were the aeroplane, submarine, truck, automobile and gasoline motors in general. The first gas attack, which was simply an adaptation of the peacetime use of the chemical chlorine, inaugurated the change. Gas was so new and instantly recognized as so powerful that the best brains in research among all the first class powers were put to work to develop other gases and other means of projecting them upon the enemy. The result was that in the short space of three and one-half years a number of substances were discovered, or experimented with anew, that are aiding today and will continue to aid in the future in the peaceful life of every nation.
Chlorine is even more valuable than ever as a disinfectant and water purifier. It is the greatest bleaching material in the world, and has innumerable other uses in the laboratory. Chloropicrin, cyanogen chloride and cyanogen bromide are found to be very well adapted to the killing of weevil and other similar insect destroyers of grain. Hydrocyanic acid gas is the greatest destroyer today of insect pests [Pg 428] that otherwise would ruin the beautiful orange and lemon groves of California and the South.
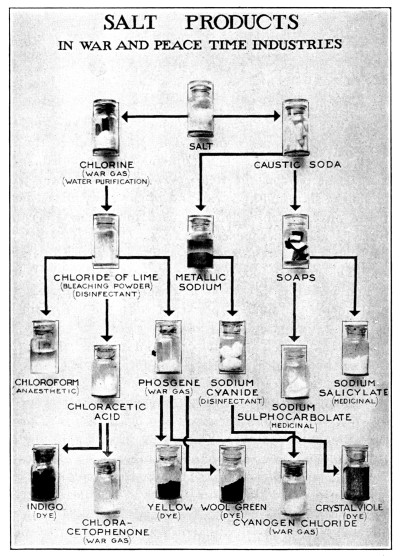
Fig. 120.
Phosgene, so extensively used in the war both in cloud gas and in shell, is finding an ever increasing use in the making of brilliant dyes—pinks, greens, blues and violets. On account of its cheapness and simplicity of manufacture, it has great possibilities in the [Pg 429] destruction of rodents such as rats around wharves, warehouses and similar places that are inaccessible to any other means of reaching those pests. Since phosgene is highly corrosive of steel, iron, copper and brass, it cannot be used successfully in places where those metals are present.
Instead of phosgene for killing rodents and the like in storehouses and warehouses, cyanogen bromide has been developed. This is a solid and can be burned like an ordinary sulphur candle. It is much safer for the purpose of fumigating rooms and buildings than is hydrocyanic acid gas when so used. This is for the reason that cyanogen bromide is an excellent lachrymator in quantities too minute to cause any injury to the lungs. It will thus give warning to anyone attempting to enter a place where some of the gas may still linger.
Among tear gases, the new chloracetophenone, a solid, is perhaps the greatest of all. When driven off by heat it first appears as a light bluish colored cloud. This cloud is instantly so irritating to the eyes that within a second anyone in the path of the cloud is temporarily blinded. It causes considerable smarting and very profuse tears which even in the smallest amount continue for two to five minutes. In greater quantities it would continue longer. So far as can be ascertained, it is absolutely harmless so far as any permanent injuries are concerned.
Considering that it is instantly effective, that minute quantities are unbearable to the eyes, that it can be put in hand grenades or other small containers and driven off by a heating mixture which will not ignite even a pile of papers, and that it needs no explosion to burst the grenade (all that is used is a light cap, set off by the action of the spring, sufficient to ignite the burning charge), the future will see every police department in the land outfitted with chloracetophenone or other similar grenades. Every sheriff’s office, every jail and every penitentiary will have a supply of them. No jail breaking, no lynching, no rioting can succeed where these grenades are available. Huge crowds can be set to weeping instantly so that no man can see and no mob will continue once it is blinded with irritating tears. More than that, it is an extremely difficult gas to keep out of masks, ordinary masks of the World War being entirely useless against it. [Pg 430]
The same is true of diphenylaminechlorarsine. This is not a tear gas but it is extraordinarily irritating to the lungs, throat and nose, where it causes pains and burning sensations, and in higher concentrations vomiting. It is hardly poisonous at all so that it is extremely difficult to get enough to cause danger to life. This is mentioned because of its possible use for the protection of bank vaults, safes, and strong rooms generally.
There are many other gases that can be used for this same purpose. It is presumed that gases that are not powerful enough to kill are the ones desired, and there are half a dozen at least that can be so used. If desired deadly gases can just as readily be used. Already a number of inventors are at work on the problem, with some plans practically completely worked out and models made.
It has been suggested that one of these gases could be used by trappers in trapping wild animals. Hydrocyanic acid gas may be so used. It acts quickly and is very rapidly dissipated. An animal exposed to the fumes would die quickly and the trap be safe to approach within two minutes after it was sprung. It is said that the loss from animals working their way out of traps by one means or another is nearly 20 per cent. More than this, it would meet the objections of the S. P. C. A. in that the animal would not suffer from having its limbs torn and lacerated by the trap.
Attempts are being made to attack the locust of the Philippines and the far west and the boll weevil of the cotton states of the South. So far these tests have not proven more successful than other methods, but inasmuch as the number of gases available for trial are so great and the value of success of so much importance, this research should be continued on a large scale to definitely determine whether poisonous gas can be used to eradicate these pests—especially the boll weevil.
As an interesting application of war materials to peaceful uses, we may consider the case of cellulose-acetate, known during the war as “aeroplane dope,” the material used to coat the linen covering aeroplane wings. With a little further manipulation, this cellulose-acetate, or aeroplane dope, becomes artificial silk—a silk that today is generally equal to the best natural silk—and which [Pg 431] promises in the future to become a standard product better in every way than that from the silk worm.
Fig. 121.
These few examples of the peacetime value of gas are worthy of thought from another standpoint. Being so valuable, their use in peace will not be stopped. If they are thus manufactured and used in peace, they will [Pg 432] always be available for use in war, and as the experience of the World War proved, they certainly will be so used even should anybody be foolish enough to try to abolish their use. As for this latter idea, the world might as well recognize at once that half-way measures in war simply invite disaster.
This chapter would not be complete without a brief statement of the necessity of a thoroughly developed chemical industry in the United States as a vital national necessity if the United States is to have real preparedness for a future struggle. As will be indicated a little later, no one branch of the chemical industry can be allowed to go out of existence without endangering some part of the scheme of preparedness.
Let us consider first the coal tar industry. Coal tar is a by-product of coke ovens or the manufacture of artificial gas from coal. The coal tar industry is of the utmost importance because in the coal tars are the bases of nearly all of the modern dyes, a large percentage of the modern medicines, most of the modern high explosives, a large proportion of poisonous gases, modern perfumes, and photographic materials.
A consideration of these titles alone shows how vital the coal tar industry is. The coal tar as it comes to us as a by-product is distilled, giving off at different temperatures a series of compounds called crudes. Ten of these are of very great importance. The first five are benzene, toluene, naphthalene, anthracene and phenol (carbolic acid). The second group comprises xylene, methylanthracene, cresol, carbazol and phenanthrene.
These, when treated with other chemicals, produce a series of compounds called intermediates, of which there are some 300 now known. From these intermediates by different steps are produced either dyes, high explosives, poisonous gases, pharmaceuticals, perfumes or photographic materials.
We have all heard that Germany controlled the dye industry of the world prior to the World War. A little study of the above brief statement of what is contained in the coal tar industry along with dyes will show in a measure one of the reasons why Germany felt that she could win a war against the world. That she came so desperately close to winning that war is proof of the soundness of her view. [Pg 433]
In many of the processes are needed the heavy chemicals such as chlorine, sulfuric acid, nitric acid, hydrochloric acid and the like. The alcohol industry is also of very great importance. Grain alcohol is used extensively in nearly all research problems and in very great quantities in many commercial processes such as the manufacture of artificial silk and for gasoline engines in addition to its use in compounding medicines. It is of very great importance to the Chemical Warfare Service in that from grain alcohol is obtained ethylene gas, one of the three essentials in the manufacture of mustard gas. While this ethylene may be obtained from many sources, the most available source, considering ease of transportation and keeping qualities, is in the form of grain alcohol.
Allied to the chemical industries just mentioned is the nitrate industry for making nitric acid from the nitrogen of the air. Nitrates are used in many processes of chemical manufacture and particularly in those for the production of smokeless powders. The fertilizer industry is of large importance because it deals with phosphorus, white phosphorus being not only one of the best smoke producing materials but a material that is, as stated elsewhere, of great use against men through its powerful burning qualities.
Another point not mentioned above in connection with these industries is the training of chemists, chemical engineers and the building up of plants for the manufacture of chemicals, all of which are necessary sources of supply for wartime needs. Chemists are needed in the field, in the laboratory and in manufacturing plants. The greater their number, the more efficiently can these materials be handled, and since chemicals as such will probably cause more than 50 per cent of all casualties in future wars, their value is almost unlimited.
Instead of trying to ameliorate the ravages of war, let us turn every endeavor towards abolishing all war, remembering that the most scientific nations should be the most highly civilized, and the ones most desirous of abolishing war. If those nations will push every scientific development to the point where by the aid of their scientific achievements they can overcome any lesser scientific peoples, the end of war should be in sight. [Pg 434]
However, we can never be certain that war is abolished until we convince at least a majority of the world that war is disastrous to the conqueror as well as to the conquered, and that any dispute can be settled peacefully if both parties will meet on the common ground of justice and a square deal.
[Pg 435]
The pioneer, no matter what the line of endeavor, encounters difficulties caused by his fellow-men just in proportion as the thing pioneered promises results. If the promise be small, the difficulties usually encountered are only those necessary to make the venture a success. If, however, the results promise to be great, and especially if the rewards to the inventor and those working with him promise to be considerable, the difficulties thrown in the way of the venture become greater and greater. Indeed whenever great results are promised, envy is engendered in those in other lines whose importance may be diminished, or who are so short-sighted as to be always opposed to progress.
Chemical warfare has had, and is still having, its full share of these difficulties. From the very day when chlorine, known to the world as a benign substance highly useful in sanitation, water purification, gold mining and bleaching was put into use as a poisonous gas, chemical warfare has loomed larger and larger as a factor to be considered in all future wars. Chlorine was first used in the cylinders designed for shipping it. These cylinders were poorly adapted for warfare, and made methods of preparing gas attacks extremely laborious, cumbersome and time-consuming.
It was not many months, however, until different gases began to appear in large quantities in shells and bombs, while the close of the war, 3½ years later, saw the development of gas in solid form whereby it could be carried with the utmost safety under all conditions—a solid which could become dangerous only when the heating mixture, that freed the gas, was properly ignited. [Pg 436]
While some of the chemicals developed for use in war prior to the Armistice have been made known to the world, a number of others have not. More than this, every nation of first class importance has continued to pursue more or less energetically studies into chemical warfare. These studies will continue, and we must expect that new gases, new methods of turning them loose, and new tactical uses will be developed.
Already it is clearly foreseen that these gases will be used by every branch of the Army and the Navy. While chemicals were not used by the Air Service in the last war, it was even then realized that there was no material reason why they should not have been so used. That they will be used in the future by the Air Service, and probably on a large scale, is certain. The Navy, too, will use gases, and probably on a considerable scale. Thus chemical materials as such become the most universal of all weapons of war.
Some of the poisonous gases are so powerful in minute quantities and evaporate so slowly that their liberation does not produce sufficient condensation to cause a cloud. Consequently, we have gases that cannot be seen. Others form clouds by themselves, such, for instance, as the toxic smoke candle, where the solid is driven off by heating, while still others cause clouds of condensed vapor. This brings the discussion into the realm of ordinary smokes that have no irritating and no poisonous effects.
These smokes are extremely valuable where the purpose is to form a screen, whether it be to hide the advance of troops or to cut off the view of observers. These smokes are equally useful on land and on sea. So great is the decrease in efficiency of the rifle or machine gun, and of artillery even when firing at troops that cannot be seen, that smoke for screening purposes will be used on every future field of battle. When firing through a screen of smoke, a man has certainly less than one-quarter the chance to hit his target that he would have were the target in plain view. Since smoke clouds may or may not be poisonous and since smoke will be used in every battle, there is opened up an unlimited field for the exercise of ingenuity in making these smoke clouds poisonous or non-poisonous at will. It also opens up an unlimited field for the well-trained chemical warfare officer who can [Pg 437] tell in any smoke cloud whether gas be present and whether, if present, it is in sufficient concentration to be dangerous.
At the risk of repetition, it is again stated that there is no gas that will kill or even permanently injure in any quantity that cannot be detected. For every gas, there is a certain minimum amount in each cubic foot of air that is necessary to cause any injury. In nearly all gases, this minimum amount is sufficient to be readily noticeable by a trained chemical warfare officer through the sense of smell.
It would be idle to attempt to enumerate the ways and means by which chemicals will be used in the future. In fact, one can hardly conceive of a situation where gas or smoke will not be employed, for these materials may be liquids or solids that either automatically, upon exposure to the air, turn into gas, or which are pulverized by high explosive, or driven off by heat. This varied character of the materials enables them to be used in every sort of artillery shell, bomb or other container carried to the field of battle.
Some of the gases are extremely powerful as irritants to the nose and throat in very minute quantities, while at the same time being highly poisonous in high concentrations. Diphenylchloroarsine, used extensively by the Germans in high explosive shell, is more poisonous than phosgene, the most deadly gas in general use in the past war. In addition, it has the quality of causing an intolerable burning sensation in the nose, throat, and lungs, in extremely minute quantities. This material can be kept out of masks only by filters, whereas true gases are taken out by charcoal and chemical granules.
There is still another quality which helps make chemical warfare the most powerful weapon of war. Gas is the only substance used in war which can be counted on to do its work as efficiently at night as in the daytime. Indeed, it is often more effective at night than in the daytime, because the man who goes to sleep without his mask on, who is careless, who loses his mask, or who becomes excited in the darkness of night, becomes a casualty, and the past war showed that these casualties were decidedly numerous even when the troops knew almost to the minute the time the gas would arrive. [Pg 438]
Accordingly, chemical warfare is an agency that must not only be reckoned with by every civilized nation in the future, but is one which civilized nations should not hesitate to use. When properly safe-guarded with masks and other safety devices, it gives to the most scientific and most ingenious people a great advantage over the less scientific and less ingenious. Then why should the United States or any other highly civilized country consider giving up chemical warfare? To say that its use against savages is not a fair method of fighting, because the savages are not equipped with it, is arrant nonsense. No nation considers such things today. If they had, our American troops, when fighting the Moros in the Philippine Islands, would have had to wear the breechclout and use only swords and spears.
Notwithstanding the opposition of certain people who, through ignorance or for other reasons, have fought it, chemical warfare has come to stay, and just in proportion as the United States gives chemical warfare its proper place in its military establishment, just in that proportion will the United States be ready to meet any or all comers in the future, for the United States has incomparable resources in the shape of the crude materials—power, salt, sulfur and the like—that are necessary in the manufacture of gases.
If, then, there be developed industries for manufacturing these gases in time of war, and if the training of the army in chemical warfare be thorough and extensive, the United States will have more than an equal chance with any other nation or combination of nations in any future war.
It is just as sportsman-like to fight with chemical warfare materials as it is to fight with machine guns. The enemy will know more or less accurately our chemical warfare materials and our methods, and we will have the same information about the enemy. It is thus a matching of wits just as much as in the days when the Knights of the Round Table fought with swords or with spears on horseback. The American is a pure sportsman and asks odds of no man. He does ask, though, that he be given a square deal. He is unwilling to agree not to use a powerful weapon of war when he knows that an outlaw nation would use it against him if that outlaw nation could achieve success by so doing. How much [Pg 439] better it is to say to the world that we are going to use chemical warfare to the greatest extent possible in any future struggle. In announcing that we would repeat as always that we are making these preparations only for defense, and who is there who dares question our right to do so?
[Pg 440]
[Pg 441]
Footnotes:
[1] This chapter originally appeared in Science, Vol. 49, pp. 412-417 (1919).
[2] Popular Science Review, 3, 176 (1864).
[3] Trans. Royal Scottish Soc. Arts, 4, Appendix O, 198 (1854).
[4] In the mixtures the percentages indicate proportions by weight.
[5] Succeeded Dr. John Johnson who went to the National esearch Council.
[6] At first Lt. Col. J. F. Norris was in charge of all chemical research. About December, 1917, it was divided into Offense and Defense, and Lt. Col. Lamb was placed in charge of Defense. When Col. Norris went to England as Liaison Officer, Dr. Jones took his place.
[7] At first Lt. Col. J. F. Norris was in charge of all chemical research. About December, 1917, it was divided into Offense and Defense, and Lt. Col. Lamb was placed in charge of Defense. When Col. Norris went to England as Liaison Officer, Dr. Jones took his place.
[8] At first Lt. Col. J. F. Norris was in charge of all chemical research. About December, 1917, it was divided into Offense and Defense, and Lt. Col. Lamb was placed in charge of Defense. When Col. Norris went to England as Liaison Officer, Dr. Jones took his place.
[9] In the early organization of the Bureau of Mines, Dr. Yandall Henderson was in charge of the Medical Sciences. Associated with him were Dr. F. P. Underhill, in charge of Therapeutic Research; Major M. C. Winternitz, in c of Pathological Research and Captain E. K. Marshall in charge of Pharmacological Research. About May 1, 1918, Pharmacological Research became so extensive that the Section was made into two, with Marshall and Loevenhart in charge, while Dr. Hunt was appointed special adviser on pharmacological problems. When the transfer to the War Department was made, Henderson, Underhill, Winternitz and Marshall were transferred to the Medical Division.
[10] Lt. Col. McPherson was formerly in charge, and was later ransferred to Ordnance.
[11] This Section was originally under H. H. Clark. Later it was split into two, with Clark and Fogler in charge, and finally consolidated under ogler.
[12] J. Ind. Eng. Chem., 11, 93 (1919).
[13] N.C. is a mixture of 80 per cent chloropicrin and 20 per cent stannic chloride.
[14] See the Pathology of War Gas Poisoning, 1920, Yale Press.
[15] See Medical Aspects of Mustard Gas Poisoning, 1919, C. O. Mosby Co.
[16] Story of the First Gas Regiment, James T. Addison. Houghton Mifflin Co., 1919.
[17] Norris, J. Ind. Eng. Chem., 11, 828 (1919).
[18] J. Am. Chem. Soc. 41, 1414 (1919).
[19] Norris, J. Ind. Eng. Chem., 11, 821 (Sept., 1919).
[20] J. Ind. Eng. Chem., 11, 292 (1919).
[21] Marshall, Lynch and Smith, J. Pharmacal, 12, 291-301 (1918).
[22] J. Pharmacol., 13, 1 (1919).
[23] Norris, J. Ind. Eng. Chem., 11, 825 (1919).
[24] Complete details of this work may be found in J. Ind. Eng. Chem., 12, 213 (1920).
[25] So-called “Triplex” glass.
[26] J. Ind. Eng. Chem., 11, 185 (1919).
[27] The basis of this chapter is the series of articles by Lamb and co-workers which appeared in the J. Ind. Eng. Chem. for 1919.
[28] Bancroft (J. Phys. Chem. 24, 127, 201, 342 [1920]) gives a comprehensive review of “Charcoal before the War.”
[29] Part of this section is quoted from “Armies of Industry,” by Crowell and Wilson, Yale Univ. Press.
[30] Which, however, was never used on the battlefield.
[31] See Fieldner and others, J. Ind. Eng. Chem., 11, 519 (1919).
[32] Taken from Fieldner’s article mentioned above.
[33] While it is a well known fact that black smoke is not as efficient as white smoke for screening purposes, the reason for this fact is not clear.
[34] This ultra-microscope is described in J. Am. Chem. Soc. 41, 312 (1919).
[35] Maximum concentration obtainable.
[36] Maximum concentration obtainable.
[37] Maximum concentration obtainable.
[38] Maximum concentration obtainable.
[39] Maximum concentration obtainable.
[40] Maximum concentration obtainable.
[41] Maximum concentration obtainable.
[42] Maximum concentration obtainable.
[43] This material is adapted from a lecture by Gen. Fries before the students of the General Staff College, in Washington, May 11, 1921.
Transcriber’s Notes:
The cover image was created by the transcriber, and is in the public domain.
The illustrations have been moved so that they do not break up paragraphs and so that they are next to the text they illustrate.
Typographical and punctuation errors have been silently corrected.